Question: please show all work. all answers shown are incorrect. My molar ratio is 1.200 Methanol (C) is formed from carbon monoxide (A) and hydrogen (B)


please show all work. all answers shown are incorrect. My molar ratio is 1.200
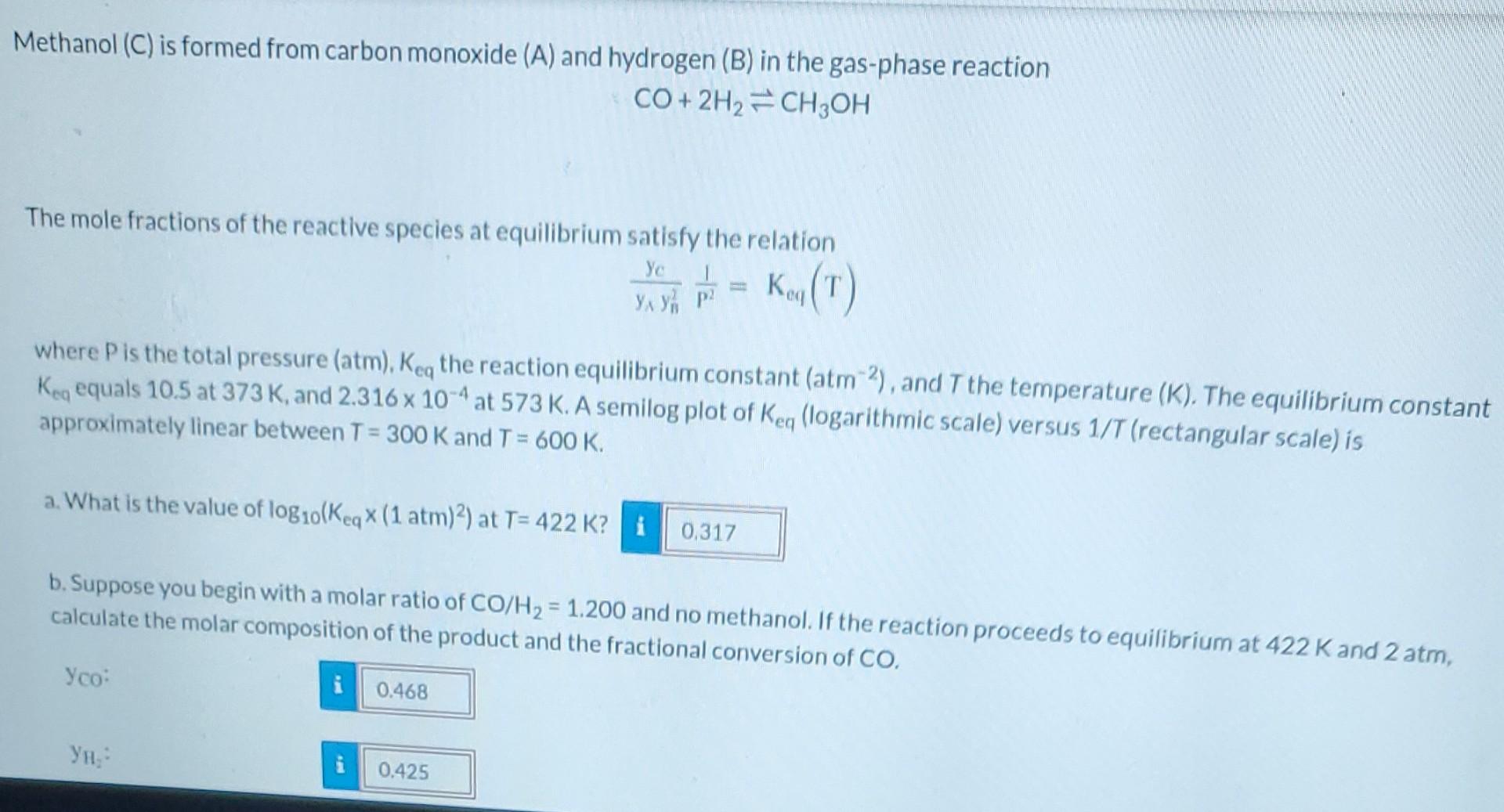
Methanol (C) is formed from carbon monoxide (A) and hydrogen (B) in the gas-phase reaction CO+2H CH3OH The mole fractions of the reactive species at equilibrium satisfy the relation Ye P/2 Keg (T) where P is the total pressure (atm), Keq the reaction equilibrium constant (atm 2), and T the temperature (K). The equilibrium constant Keq equals 10.5 at 373 K, and 2.316 x 10-4 at 573 K. A semilog plot of Keq (logarithmic scale) versus 1/T (rectangular scale) is approximately linear between T = 300 K and T = 600 K. a. What is the value of log10(Keq x (1 atm)2) at T=422 K? i 0,317 b. Suppose you begin with a molar ratio of CO/H = 1.200 and no methanol. If the reaction proceeds to equilibrium at 422 K and 2 atm, calculate the molar composition of the product and the fractional conversion of CO. Yco: 0.468 YH: 0.425 &200 b. Suppose you begin with a molar ratio of CO/H = 1.200 and no methanol. If the reaction proceeds to equilibrium at 422 K and 2 atm, calculate the molar composition of the product and the fractional conversion of CO. i Yco: 0.468 0.425 0.1066 0.1855 Y CHOH Fractional Conversion: M. c. Repeat the calculation for a molar ratio of CO/H = 1.450. i 6.54 Yco: ,: 0.367 YCH, OH i 0.042 Fractional Conversion: i 14.52
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


