Question: ALSO: predict whether or not the reactions will be spontaneous or not Calculate the change in Gibbs energy for each of the sets of rH,rS,

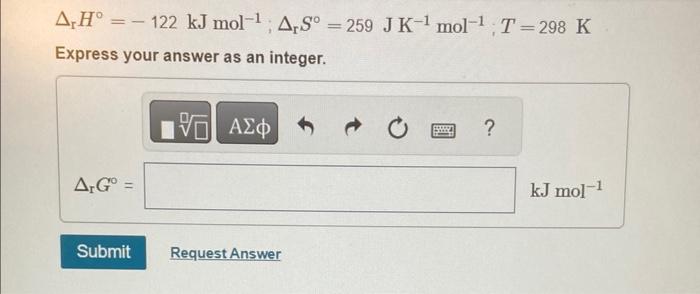
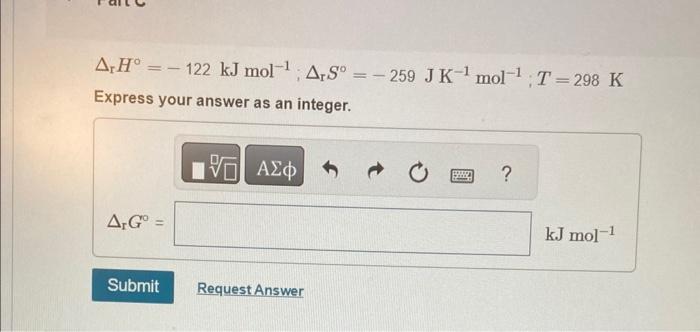
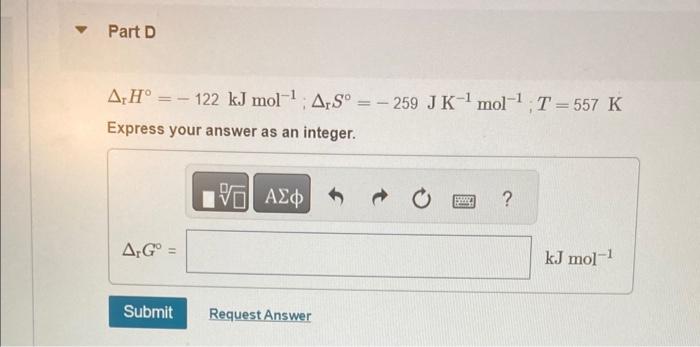
Calculate the change in Gibbs energy for each of the sets of rH,rS, and T. rH=122kJmol1;rS=259JK1mol1;T=298K Express your answer as an integer. rH=122kJmol1;rS=259JK1mol1;T=298K Express your answer as an integer. rH=122kJmol1;rS=259JK1mol1;T=557K Express your answer as an integer
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


