Question: am i solving this by how close together the molecules are? Note: all of the molecules are neutral, and you may assume non substance electrostatic
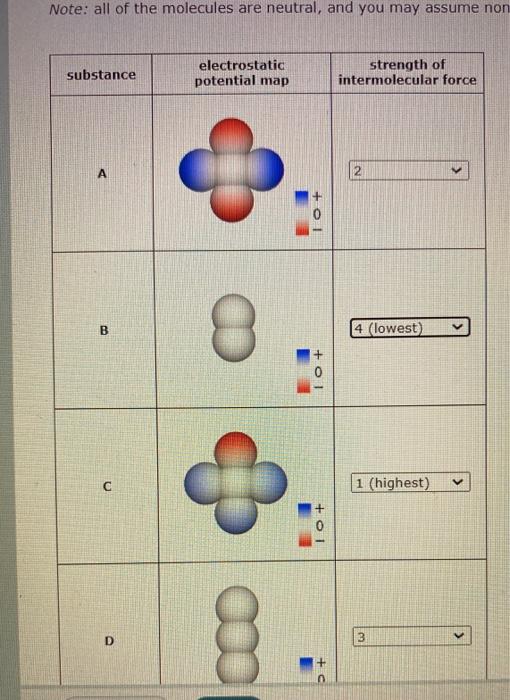

Note: all of the molecules are neutral, and you may assume non substance electrostatic potential map strength of intermolecular force A 2 + 01 B 8 4 (lowest) +O1 V 1 (highest) 0 D 8. 8. 3 Molecules of four imaginary substances are sketched in the table below. Each sketch is shaded to show the electrostatic potential at the surface of the molecule. Rank these substances in decreasing order of the strength of the intermolecular forces in them In other words, choose 1 next to the substance in which the molecules exert the strongest intermolecular forces on each other. Choose 2 next to the substance in which the molecules exert the second strongest intermolecular forces on each other, and so forth. Note: all of the molecules are neutral, and you may assume none of them experience hydrogen bonding
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


