Question: Ammonia forms a complex ion with A g + , N i 2 + , and Z n 2 + , but does not form
Ammonia forms a complex ion with and but does not form a complex
with or
Compare the solubility of the following compounds in to the solubility in
pure water
A less soluble in than in water
B equally soluble in and water
C more soluble in than in water
D less soluble in than in water
E more soluble in than in water
less soluble in than in water
less soluble in than in water
more soluble in than in water
more soluble in than in water
equally soluble in and water
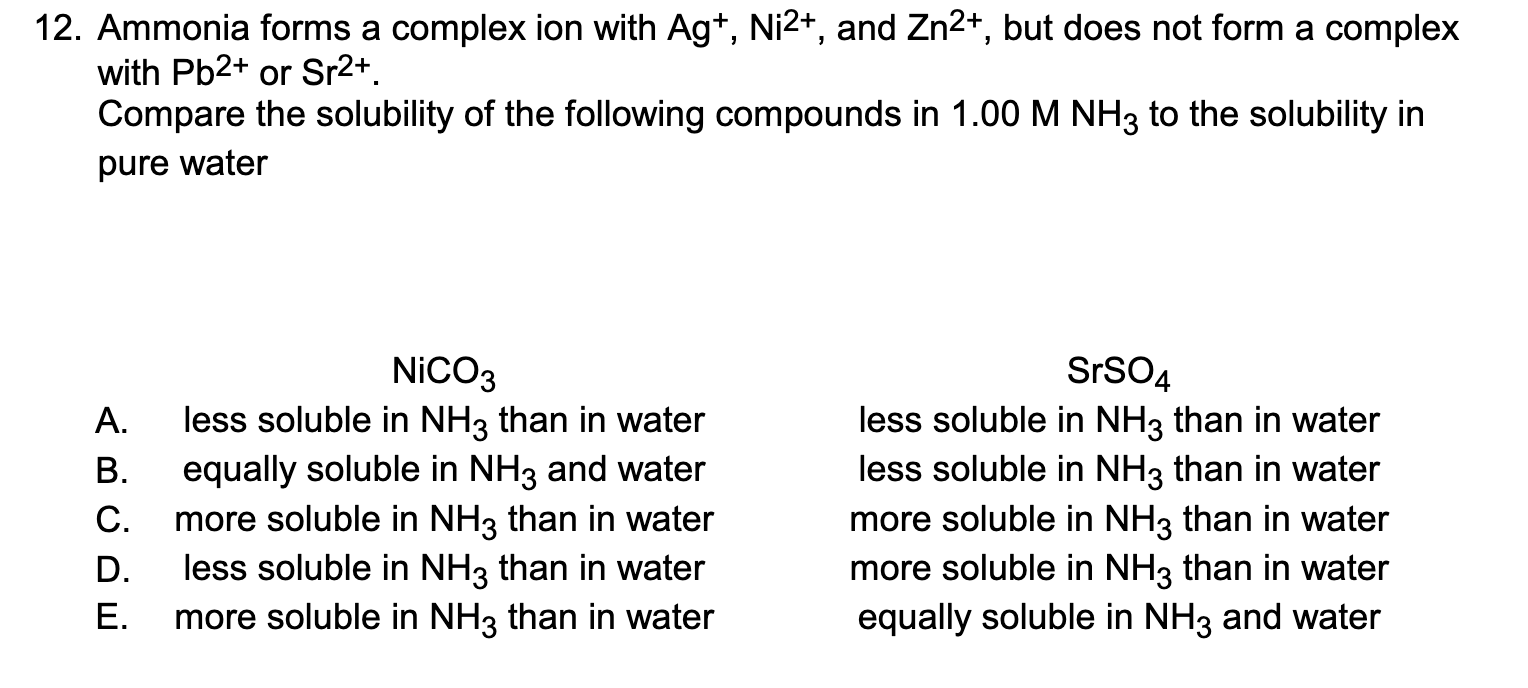
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


