Question: Amphetamine is a weak base (pKa10) while ibuprofen is a relatively weak acid (pKa4.4). Amphetamine lbuprofen (i) Discuss the effect of pH on absorption of
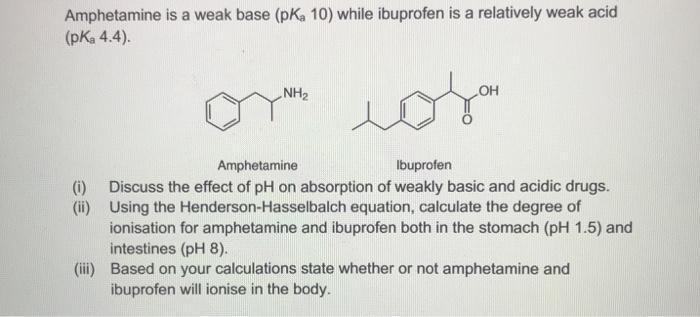
Amphetamine is a weak base (pKa10) while ibuprofen is a relatively weak acid (pKa4.4). Amphetamine lbuprofen (i) Discuss the effect of pH on absorption of weakly basic and acidic drugs. (ii) Using the Henderson-Hasselbalch equation, calculate the degree of ionisation for amphetamine and ibuprofen both in the stomach (pH1.5) and intestines ( pH8). (iii) Based on your calculations state whether or not amphetamine and ibuprofen will ionise in the body
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


