Question: An adiabatic constant volume box is divided into left and right by a plate, and ideal gas A, B, and the initial states are shown
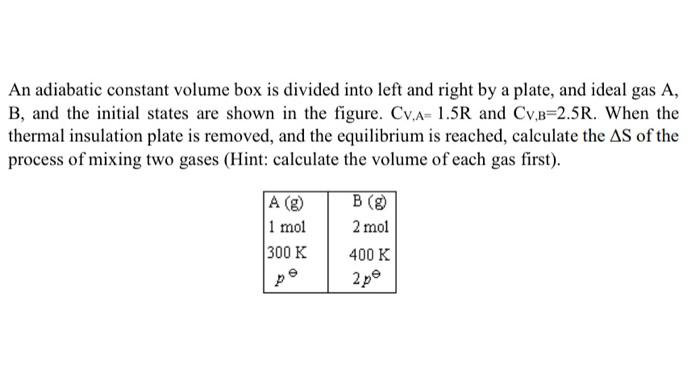
An adiabatic constant volume box is divided into left and right by a plate, and ideal gas A, B, and the initial states are shown in the figure. CV,A=1.5R and CV,B=2.5R. When the thermal insulation plate is removed, and the equilibrium is reached, calculate the S of the process of mixing two gases (Hint: calculate the volume of each gas first)
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


