Question: An aqueous potassium chloride solution is prepared by dissolving 45.9 g of KCl in 435.0 g of water. What is the freezing point (in C)
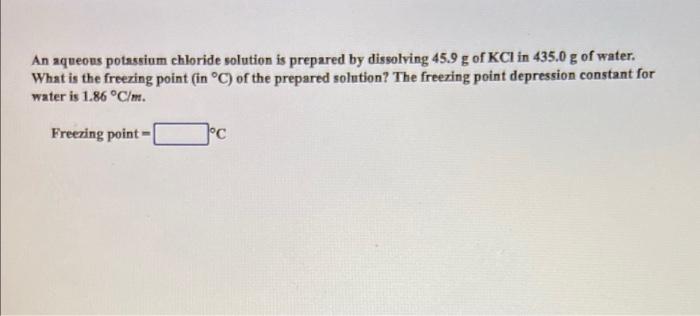
An aqueous potassium chloride solution is prepared by dissolving 45.9 g of KCl in 435.0 g of water. What is the freezing point (in C) of the prepared solution? The freezing point depression constant for water is 1.86 C/m. Freezing point 1C
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


