Question: An atom exists in three energy levels: Level 1 at 0J Level 2 at 2.01019J Level 3 at 4.841019J. Approximately what wavelength of light (in
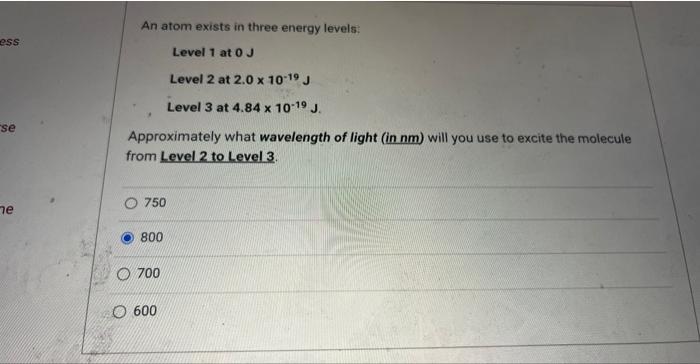

An atom exists in three energy levels: Level 1 at 0J Level 2 at 2.01019J Level 3 at 4.841019J. Approximately what wavelength of light (in nm) will you use to excite the molecule from Level 2 to Level 3. 750 800 700 600 Electromagnetic radiation with a wavelength of 720nm is not visible to the human eye. The frequency of this light is Hz. 4.551014 4.17105 5.711014 4.171014 8.221011
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


