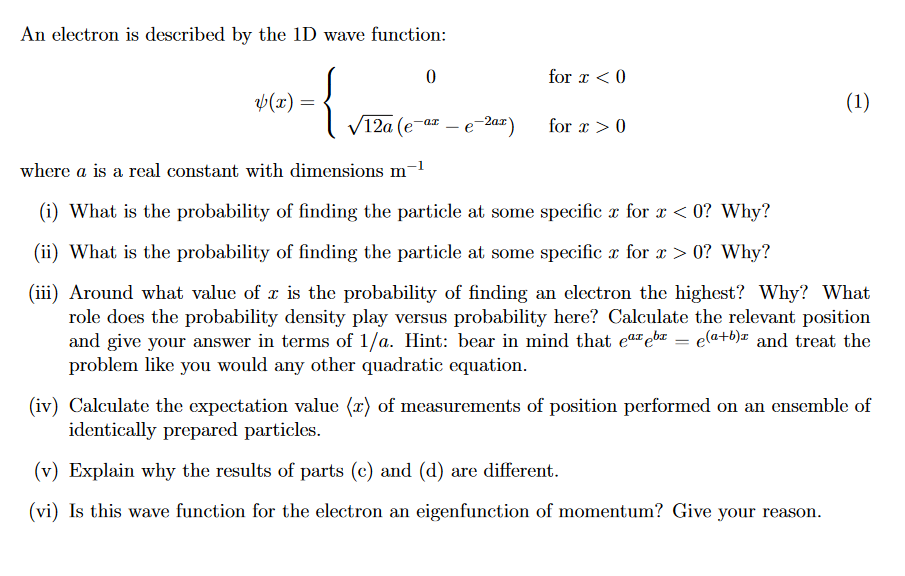
Question: An electron is described by the 1 D wave function: ( x ) = { 0 f o r x 0 1 2 a 2
An electron is described by the D wave function:
where is a real constant with dimensions
i What is the probability of finding the particle at some specific for Why?
ii What is the probability of finding the particle at some specific for Why?
iii Around what value of is the probability of finding an electron the highest? Why? What
role does the probability density play versus probability here? Calculate the relevant position
and give your answer in terms of Hint: bear in mind that and treat the
problem like you would any other quadratic equation.
iv Calculate the expectation value :: of measurements of position performed on an ensemble of
identically prepared particles.
v Explain why the results of parts c and d are different.
vi Is this wave function for the electron an eigenfunction of momentum? Give your reason.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


