Question: An equilibrium flash separator operating at steady state is fed with water, ethanol, and n-propanol mixture with mole fraction of 0.3, 0.3, and 0.4, respectively.
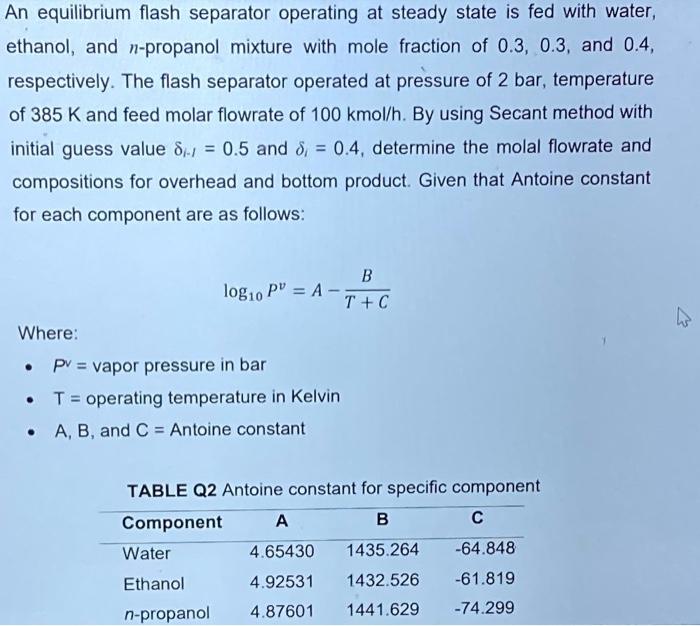
An equilibrium flash separator operating at steady state is fed with water, ethanol, and n-propanol mixture with mole fraction of 0.3, 0.3, and 0.4, respectively. The flash separator operated at pressure of 2 bar, temperature of 385 K and feed molar flowrate of 100 kmol/h. By using Secant method with initial guess value dil = 0.5 and 8) = 0.4, determine the molal flowrate and compositions for overhead and bottom product. Given that Antoine constant for each component are as follows: = 4 B log10 PV = A - T+C Where: pv = vapor pressure in bar T = operating temperature in Kelvin A, B, and C = Antoine constant . TABLE Q2 Antoine constant for specific component Component A B C Water 4.65430 1435.264 -64.848 Ethanol 4.92531 1432.526 -61.819 n-propanol 4.87601 1441.629 -74.299
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


