Question: An example of two parameter sets for both water (normal boiling point of water is 100 C) and ethanol (normal boiling point of ethanol is
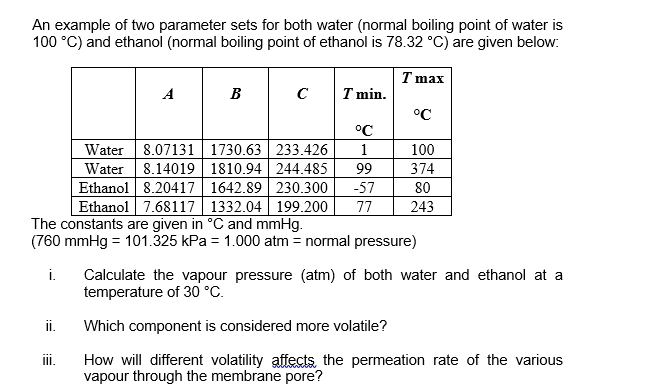
An example of two parameter sets for both water (normal boiling point of water is 100 C) and ethanol (normal boiling point of ethanol is 78.32 C) are given below: 99 T max A B I min. C C Water 8.07131 | 1730.63 233.426 1 100 Water 8.14019 1810.94244.485 374 Ethanol 8.20417 1642.89 230.300 -57 80 Ethanol 7.68117 1332.04 199 200 77 243 The constants are given in C and mmHg. (760 mmHg = 101.325 kPa = 1.000 atm = normal pressure) i. Calculate the vapour pressure (atm) of both water and ethanol at a temperature of 30 C. ii. Which component is considered more volatile? iii. How will different volatility affects the permeation rate of the various vapour through the membrane pore
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


