Question: An experimental study was conducted on a mixture containing equimolar amounts of ethanol (1) and n-hexane (2) compounds, undergoing a separation process under conditions of
An experimental study was conducted on a mixture containing equimolar amounts of ethanol (1) and n-hexane (2) compounds, undergoing a separation process under conditions of 318.15 K and 65 kPa. The following data were obtained for this system at a temperature of 318.15 K:
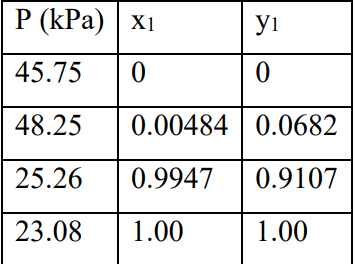
Utilize the data in the table to determine the appropriate coefficients for two fixed Margules equations.
a) Using a suitable, qualified computer program, demonstrate whether the given mixture will undergo phase separation into two phases as a result of a flash operation at 65 kPa and 318.15 K conditions.
b) If phase separation occurs as a result of the flash operation, calculate the fractional amounts and compositions of the streams exiting the unit. (HINT: To calculate these values, the relevant equations need to be simultaneously solved. Therefore, perform your solution using a MATLAB program.)
c) Using a MATLAB program, appropriately plot the Pxy phase diagram based on the two-fixed Margules equations, as well as the ln i, GE/RT, and GE/(x1x2RT) graphs against x1 quantity.
NOTE: Refer to Figures 13.6, 13.7, and 13.8 for the appropriate drawing format.
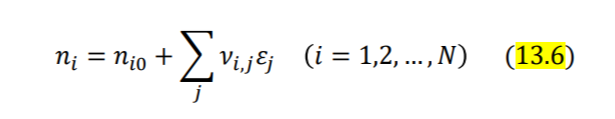
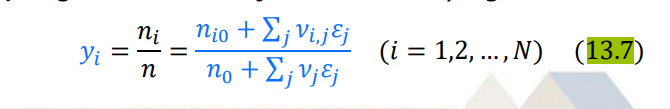
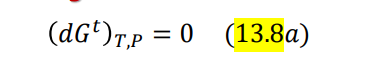

\begin{tabular}{|l|l|l|} \hline P(kPa) & x1 & y1 \\ \hline 45.75 & 0 & 0 \\ \hline 48.25 & 0.00484 & 0.0682 \\ \hline 25.26 & 0.9947 & 0.9107 \\ \hline 23.08 & 1.00 & 1.00 \\ \hline \end{tabular} ni=ni0+jvi,jj(i=1,2,,N) yi=nni=n0+jvjjni0+jvi,jj(i=1,2,,N) (dGt)T,P=0 iii=0
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


