Question: Torror endte If each reaction species changes at a different rate how can a single reaction rate be defined for that reaction? Identify which of
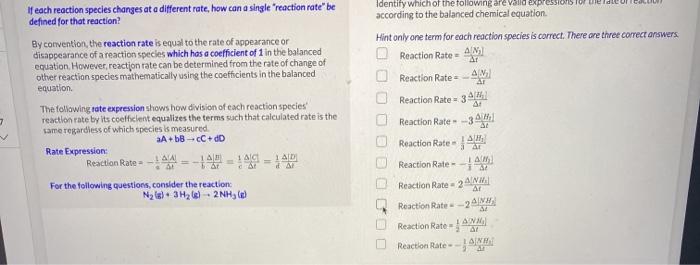
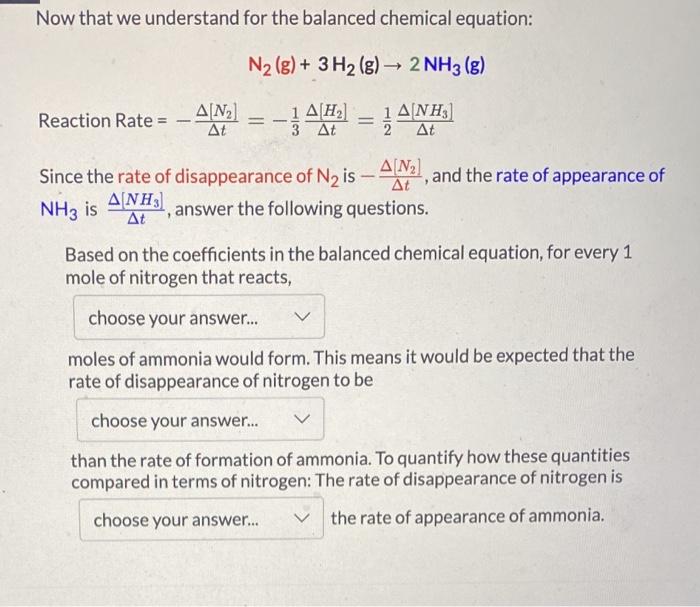
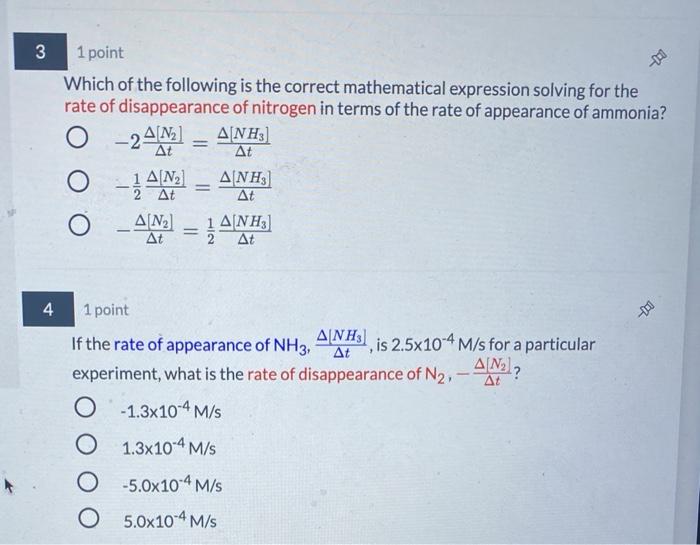
Torror endte If each reaction species changes at a different rate how can a single reaction rate" be defined for that reaction? Identify which of the following are valid expl according to the balanced chemical equation Hint only one term for each reaction species is correct. There are three correct answers AN Reaction Rate By convention, the reaction rate is equal to the rate of appearance or disappearance of a reaction species which has a coefficient of 1 in the balanced equation. However reaction rate can be determined from the rate of change of other reaction species mathematically using the coefficients in the balanced equation Reaction Rate 7 2 The following rate expression shows how division of each reaction species reaction rate by its coefficient equalizes the terms such that calculated rate is the same regardless of which species is measured A+bBC+dD Rate Expression Reaction Rate == 14b = 14 AD For the following questions, consider the reaction Ny(s) 3H2)2NH, OG DEED Reaction Rate 34/14 Reaction Rate -34 Reaction Rate Reaction Rate AM Reaction Rate - 2 ANN Reaction Rate-2 Reaction Rate = JAM Reaction Rate - - AINA Now that we understand for the balanced chemical equation: N2(g) + 3H2(g) 2NH3 (g) 2 At Reaction Rate = -4 () = -1442 1 ANH) Since the rate of disappearance of Nz is A/N2), and the rate of appearance of 3] answer the following questions. NH3 is At Based on the coefficients in the balanced chemical equation, for every 1 mole of nitrogen that reacts, choose your answer... moles of ammonia would form. This means it would be expected that the rate of disappearance of nitrogen to be choose your answer... than the rate of formation of ammonia. To quantify how these quantities compared in terms of nitrogen: The rate of disappearance of nitrogen is choose your answer... the rate of appearance of ammonia. 3 - 1 point Which of the following is the correct mathematical expression solving for the rate of disappearance of nitrogen in terms of the rate of appearance of ammonia? O A NH) At At O-AN 3] At 1ANH) At - 240] _A[N] ] At 4 At 1 point ] If the rate of appearance of NH3, is 2.5x10-4 M/s for a particular experiment, what is the rate of disappearance of N2. -AN? O -1.3x10-4 M/S 1.3x10-4 M/S O -5.0x10-4 M/S O 5.0x10-4 M/S
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


