Question: An isothermal variable-volume batch reactor was loaded with pure A and used to carry out multiple runs of the following gas-phase reaction at a pressure
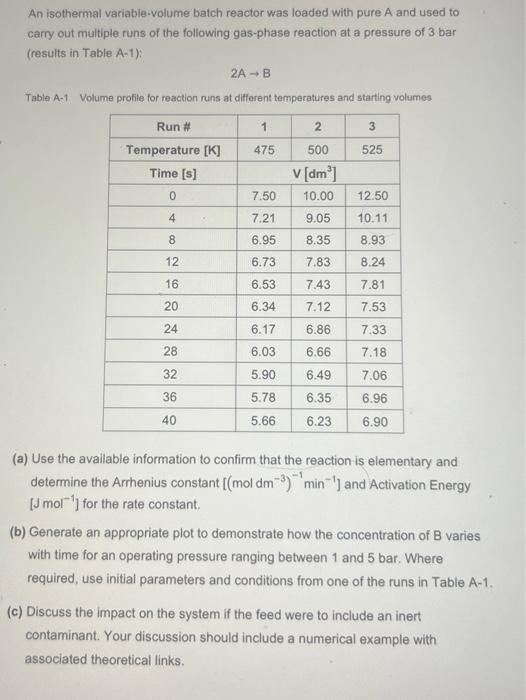
An isothermal variable-volume batch reactor was loaded with pure A and used to carry out multiple runs of the following gas-phase reaction at a pressure of 3 bar (results in Table A-1): 2AB Table A-1 Volume profile for reaction runs at different temperatures and starting volumes (a) Use the available information to confirm that the reaction is elementary and determine the Arrhenius constant [(moldm3)1min1] and Activation Energy [Jmol1] for the rate constant. (b) Generate an appropriate plot to demonstrate how the concentration of B varies with time for an operating pressure ranging between 1 and 5 bar. Where required, use initial parameters and conditions from one of the runs in Table A-1. (c) Discuss the impact on the system if the feed were to include an inert contaminant. Your discussion should include a numerical example with associated theoretical links
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


