Question: An oxygen electrode in a basic solution has a voltage of 0.5628V. If PO2=0.1atm, what is the solution pH ? From the emf series, O2+2H2O+4e=4(OH)E=+0.40V
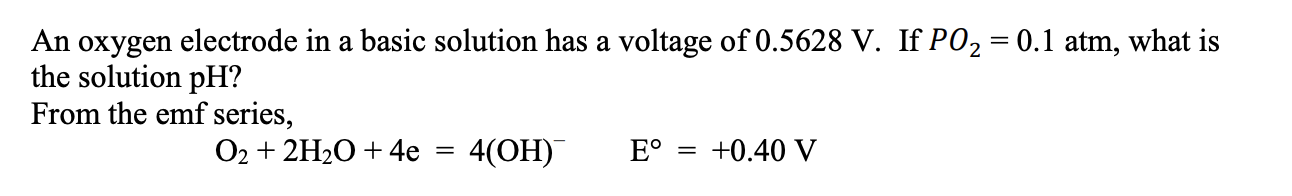
An oxygen electrode in a basic solution has a voltage of 0.5628V. If PO2=0.1atm, what is the solution pH ? From the emf series, O2+2H2O+4e=4(OH)E=+0.40V
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


