Question: An unknown compound was found to have a percent composition as follows: 47.0% potassium, 14.5% carbon, and 38.5% oxygen. What is its empirical formula? If
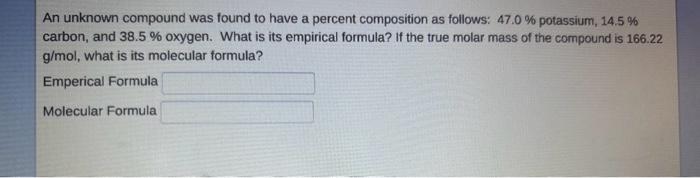
An unknown compound was found to have a percent composition as follows: 47.0% potassium, 14.5% carbon, and 38.5% oxygen. What is its empirical formula? If the true molar mass of the compound is 166.22 g/mol, what is its molecular formula? Emperical Formula Molecular Formula
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


