Question: Analysis #2: Mass Spectrometer Indicates The Unknown Compound Has A Molecular Weight Of 102.2 G/Mol. Analysis #3: Infrared Spectroscopy Indicates The Unknown Compound Has The
Analysis #2: Mass Spectrometer Indicates The Unknown Compound Has A Molecular Weight Of 102.2 G/Mol. Analysis #3: Infrared Spectroscopy Indicates The Unknown Compound Has The Following Spectra: What Is The Correct Structure Of Ch3(Ch2)2o(Ch2)2ch3?
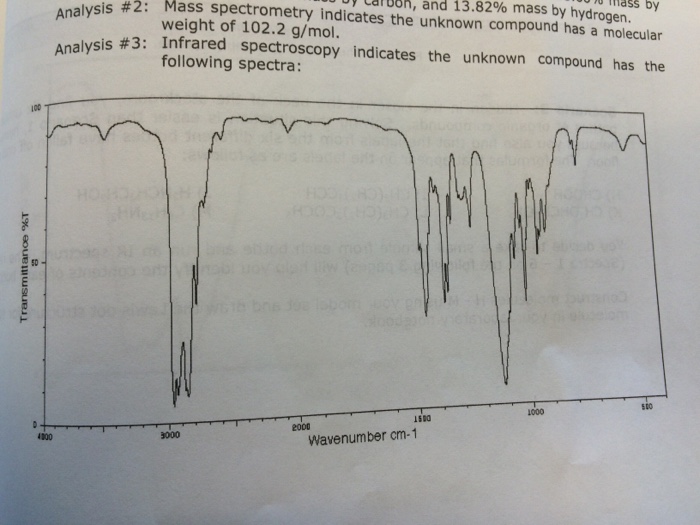
What is the correct structure of ch3(ch2)2o(ch2)2ch3?
50 Transmittance %T 100 and 13.82% mass by hydrogen. iss by Analysis #2: Mass spectrometry indicates the unknown compound has a molecular weight of 102.2 g/mol. Analysis #3: Infrared spectroscopy indicates the unknown compound has the following spectra: 4000 3000 2000 1600 1000 500 Wavenumber cm-1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


