Question: Analyzing for Tin Lab Extensive long-term research has found that treating children's teeth with fluoride significantly reduces tooth decay. When this was first discovered, toothpastes
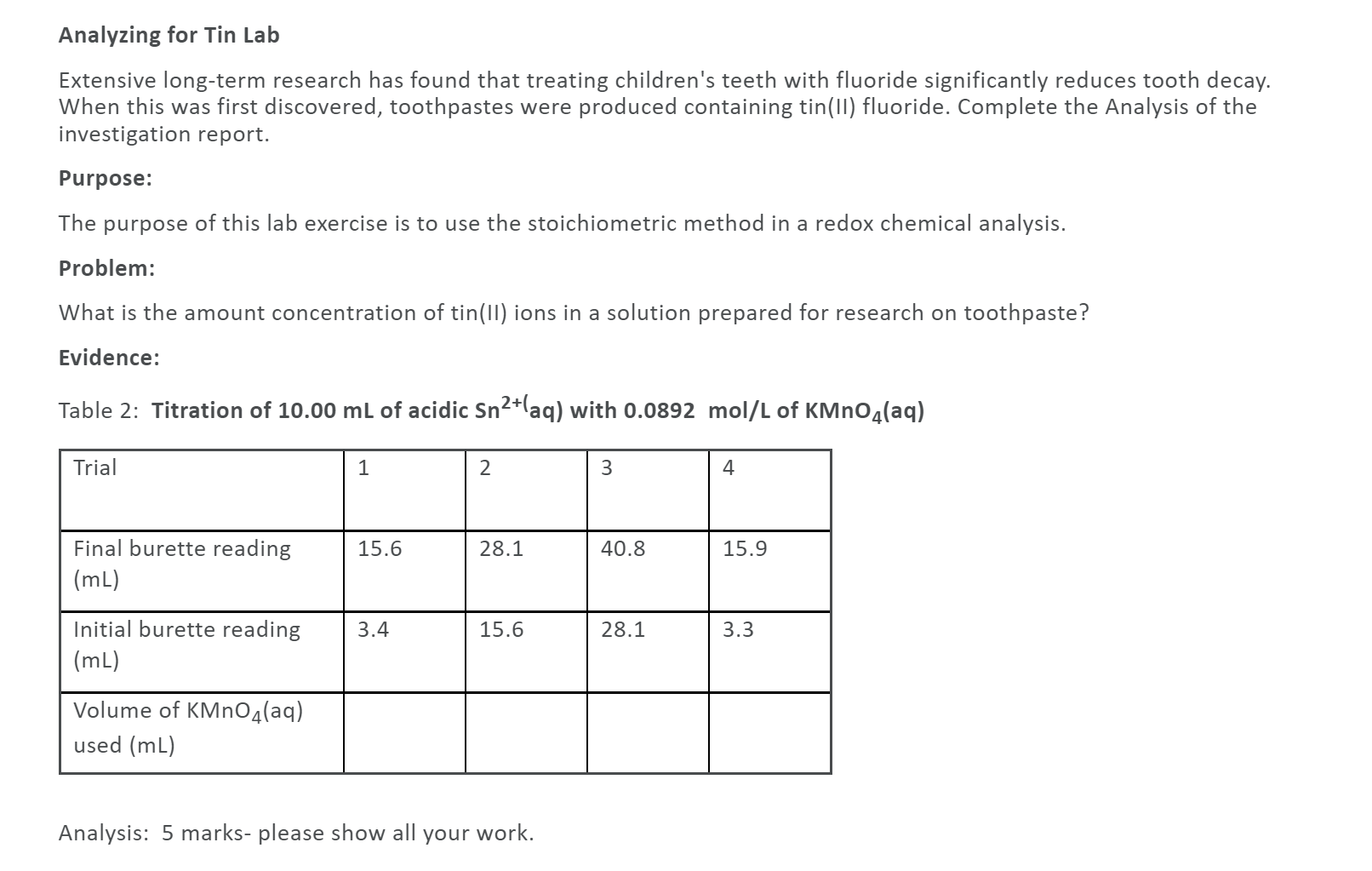

Analyzing for Tin Lab Extensive long-term research has found that treating children's teeth with fluoride significantly reduces tooth decay. When this was first discovered, toothpastes were produced containing tin(11) fluoride. Complete the Analysis of the investigation report. Purpose: The purpose of this lab exercise is to use the stoichiometric method in a redox chemical analysis. Problem: What is the amount concentration of tin(11) ions in a solution prepared for research on toothpaste? Evidence: Table 2: Titration of 10.00 mL of acidic Sn2+(aq) with 0.0892 mol/L of KMnO4(aq) Trial 1 2 3 4 15.6 28.1 40.8 15.9 Final burette reading (mL) 3.4 15.6 28.1 3.3 Initial burette reading (mL) Volume of KMnO4(aq) used (mL) Analysis: 5 marks- please show all your work. Question 2 (5 points) Analyzing for Chromium in Steel Stainless steel is a corrosion-resistant, esthetically pleasing alloy, normally composed of nickel, chromium, and iron. Complete the Analysis of the investigation report. Purpose: The purpose of this lab exercise is to use the stoichiometric method in a redox chemical analysis. Problem: What is the amount concentration of chromium (11) ions in a solution obtained in the analysis of a stainless steel alloy? Design: A standard potassium dichromate solution is used as an oxidizing agent to oxidize chromium (II) ions to chromium(III) ions in an acidic solution Evidence: Table 2: Titration of 13.00 mL of acidic Cr 2+laq) with 0.329 mol/L K2Cr2O7(aq) Trial 1 2 3 4 17.4 34.9 18.9 21.1 Final burette reading (mL) 0.00 17.4 1.5 3.6 Initial burette reading (mL) Volume of K2Cr2O7(aq) used (mL) Analysis: 5 marks- please show all your work
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


