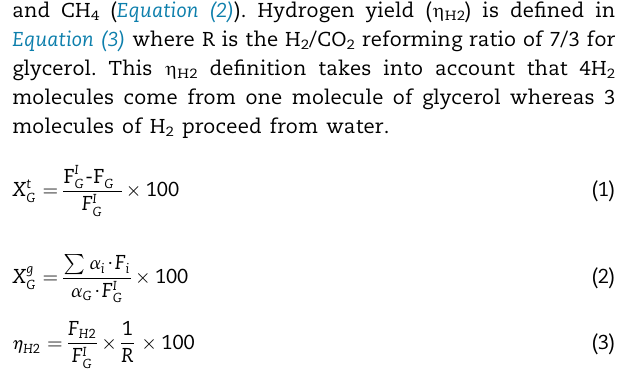
Question: and C H 4 ( Equation ( 2 ) ) . Hydrogen yield ( H 2 ) is defined in Equation ( 3 ) where
and Equation Hydrogen yield is defined in
Equation where is the reforming ratio of for
glycerol. This definition takes into account that
molecules come from one molecule of glycerol whereas
molecules of proceed from water.
This should've been a follow up question, but chegg doesn't allow replies so I'll make another one. Assuming a kghr hr basis of wt glycerol water, glycerol conversion and hydrogen yield, and exit gas stream composition of H COaccording to the study Im basing on how would the material balance equation look like? The answer I got was mol H per kg feed, as well as mol CO but I feel like the wording of the formulas confused me
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


