Question: ank the relative stability. a. 1= most stable, 3 = least stable. Two of the carbocations are so unstable/high energy they will not form. Label
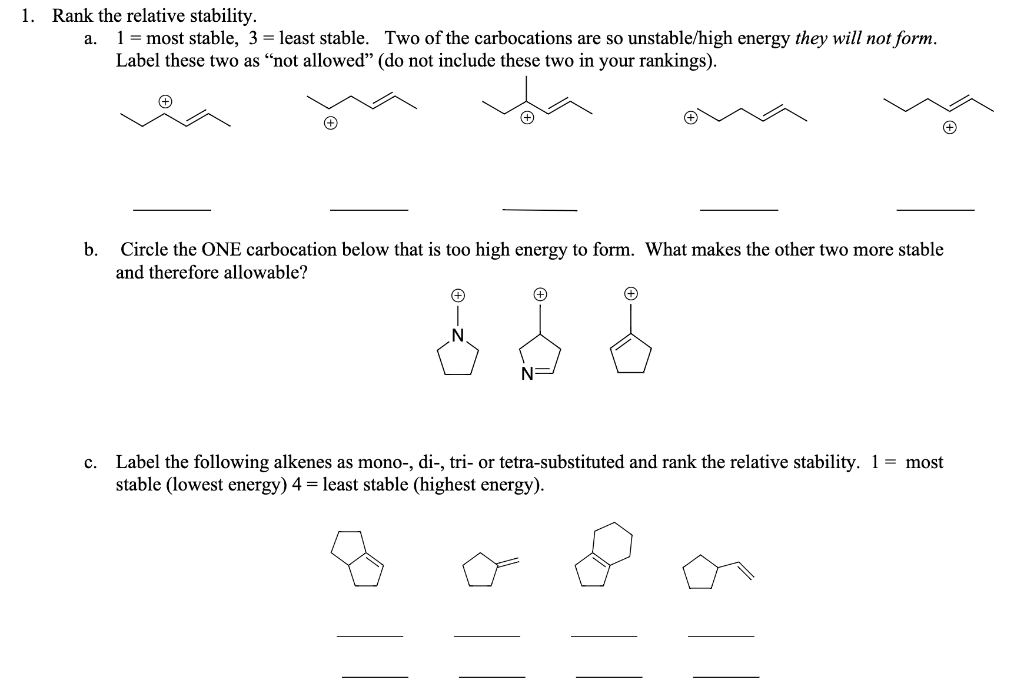
ank the relative stability. a. 1= most stable, 3 = least stable. Two of the carbocations are so unstable/high energy they will not form. Label these two as "not allowed" (do not include these two in your rankings). ( ) b. Circle the ONE carbocation below that is too high energy to form. What makes the other two more stable and therefore allowable? c. Label the following alkenes as mono-, di-, tri- or tetra-substituted and rank the relative stability. 1= most stable (lowest energy) 4= least stable (highest energy)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


