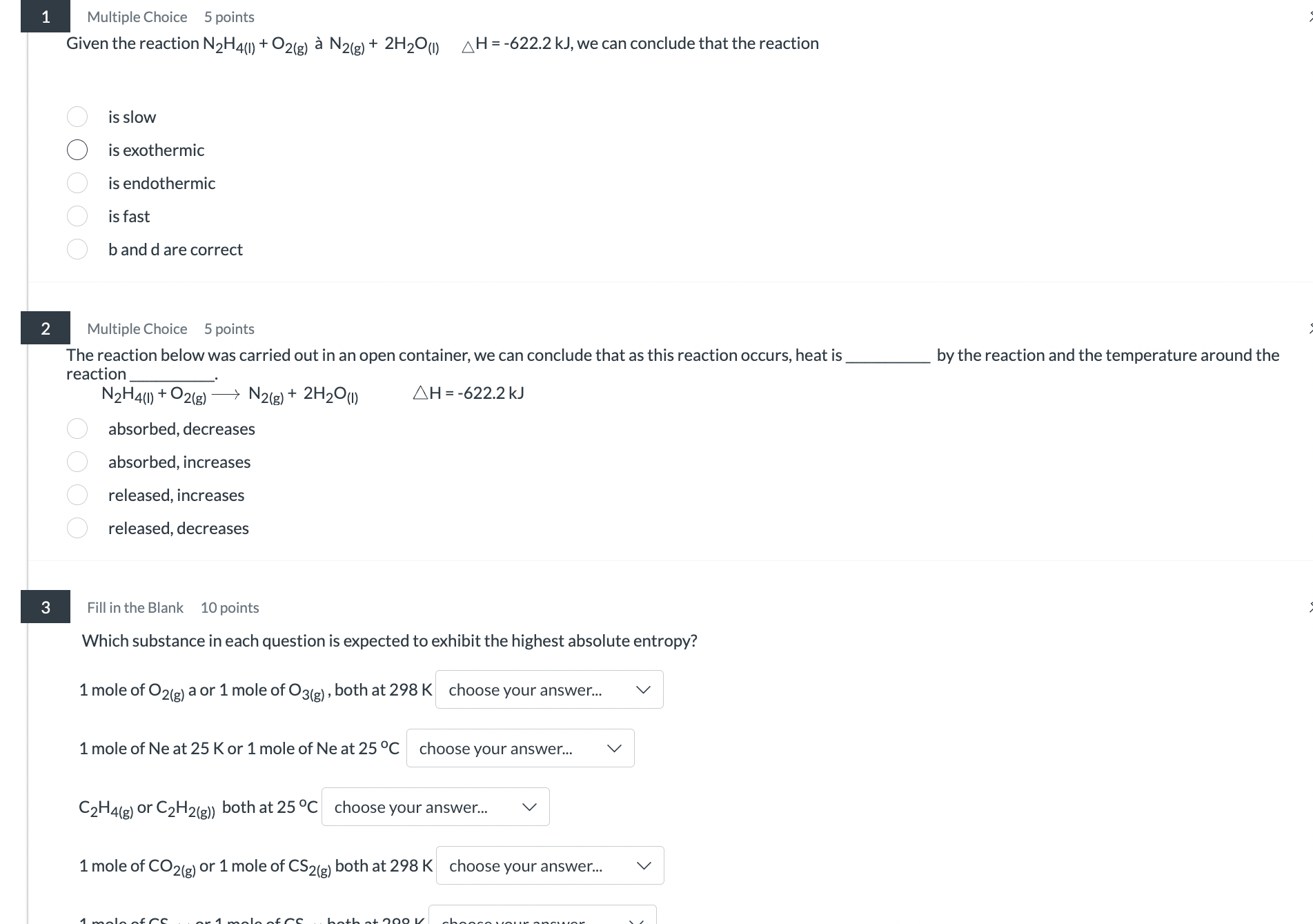
Question: answer 1 Multiple Choice 5 points Given the reaction N2H4(1) + 02(g) a N2(g) + 2H20(1) AH=-622.2 kJ, we can conclude that the reaction O
answer
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


