Question: answer all Classify each reaction as an oxidation, a reduction, or neither. Part A H2CrO4 reduction, reduction reduction, oxidation oxidation, oxidation neither Part Text: CH4CH3OH
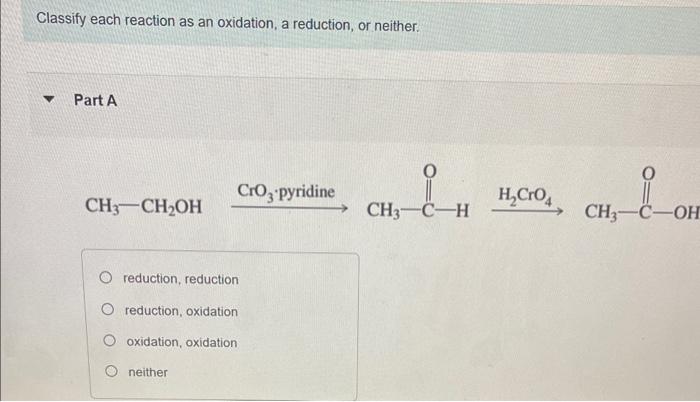
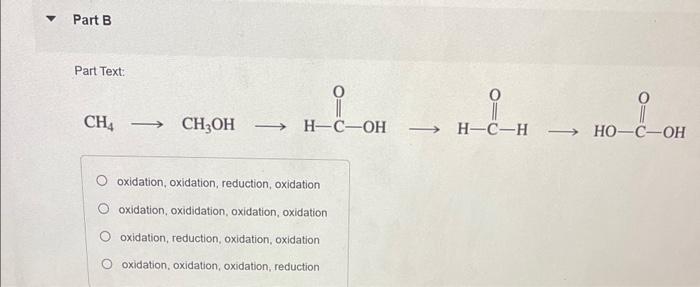
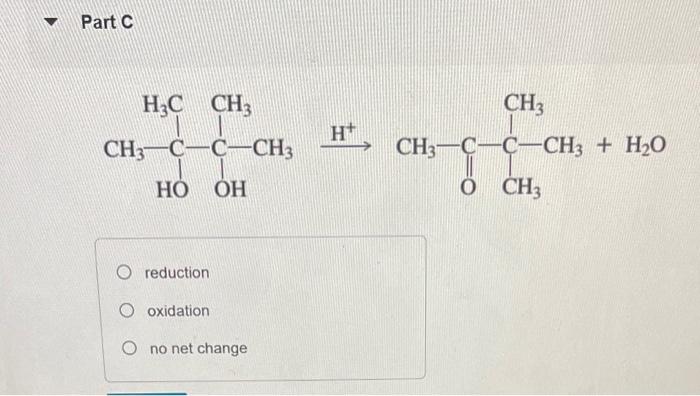
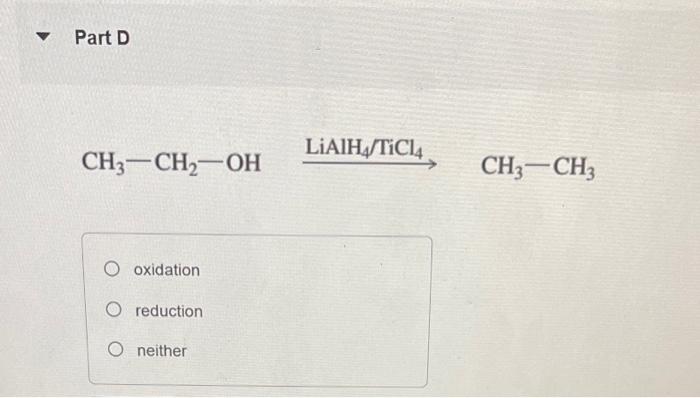
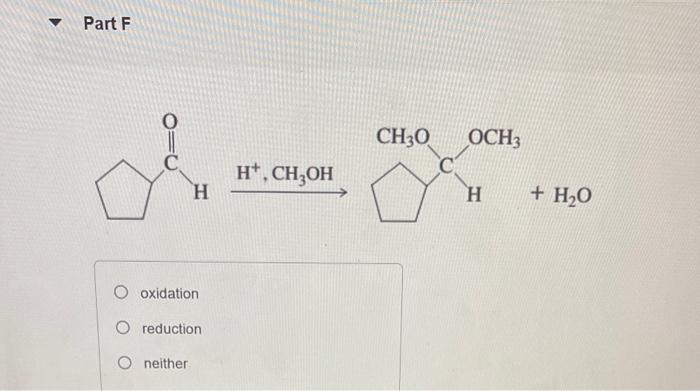
Classify each reaction as an oxidation, a reduction, or neither. Part A H2CrO4 reduction, reduction reduction, oxidation oxidation, oxidation neither Part Text: CH4CH3OH oxidation, oxidation, reduction, oxidation oxidation, oxididation, oxidation, oxidation oxidation, reduction, oxidation, oxidation oxidation, oxidation, oxidation, reduction H+ reduction oxidation no net change CH3CH2OH oxidation reduction neither Part F H+,CH3OH +H2O oxidation reduction neither
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


