Question: ANSWER ALL CORRECTLY. show the working method calculation and explain. In a 1L batch reactor, 1mol/L carbonic acid decomposes into carbon dioxide and water at
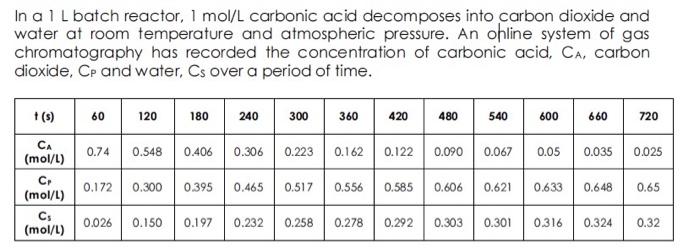
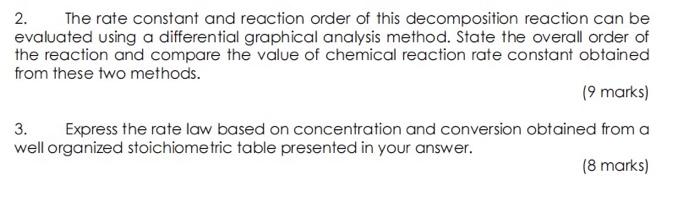
In a 1L batch reactor, 1mol/L carbonic acid decomposes into carbon dioxide and water at room temperature and atmospheric pressure. An ohline system of gas chromatography has recorded the concentration of carbonic acid, CA, carbon dioxide, Cp and water, Cs over a period of time. 2. The rate constant and reaction order of this decomposition reaction can be evaluated using a differential graphical analysis method. State the overall order of the reaction and compare the value of chemical reaction rate constant obtained from these two methods. (9 marks) 3. Express the rate law based on concentration and conversion obtained from a well organized stoichiometric table presented in your answer. ( 8 marks)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


