Question: answer all only need the correct answer SECTION A Answer the following Q1 to 27 as directed. (Each question is of 1 mark). [07] 1.
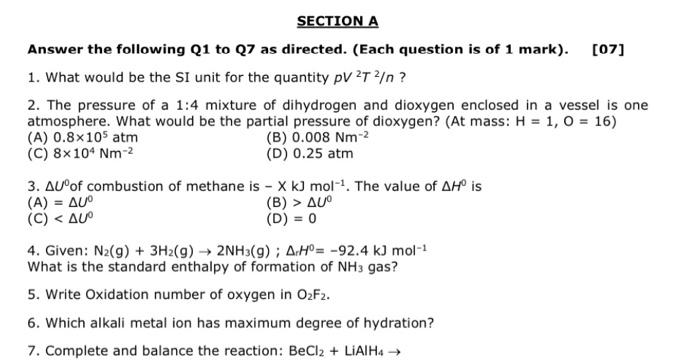
SECTION A Answer the following Q1 to 27 as directed. (Each question is of 1 mark). [07] 1. What would be the SI unit for the quantity pv 2T ? ? 2. The pressure of a 1:4 mixture of dihydrogen and dioxygen enclosed in a vessel is one atmosphere. What would be the partial pressure of dioxygen? (At mass: H = 1, 0 = 16) (A) 0.8x105 atm (B) 0.008 Nm-2 (C) 8x10 Nm-2 (D) 0.25 atm 3. AUof combustion of methane is - X k) mol-!. The value of AHO is (A) = AU (B) > AU (C)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


