Question: Answer all parts please. A gas is compressed adiabatically from 10atm and 3000K to 50atm and 5000K at the rate of 1kmol/s. It is assumed
Answer all parts please.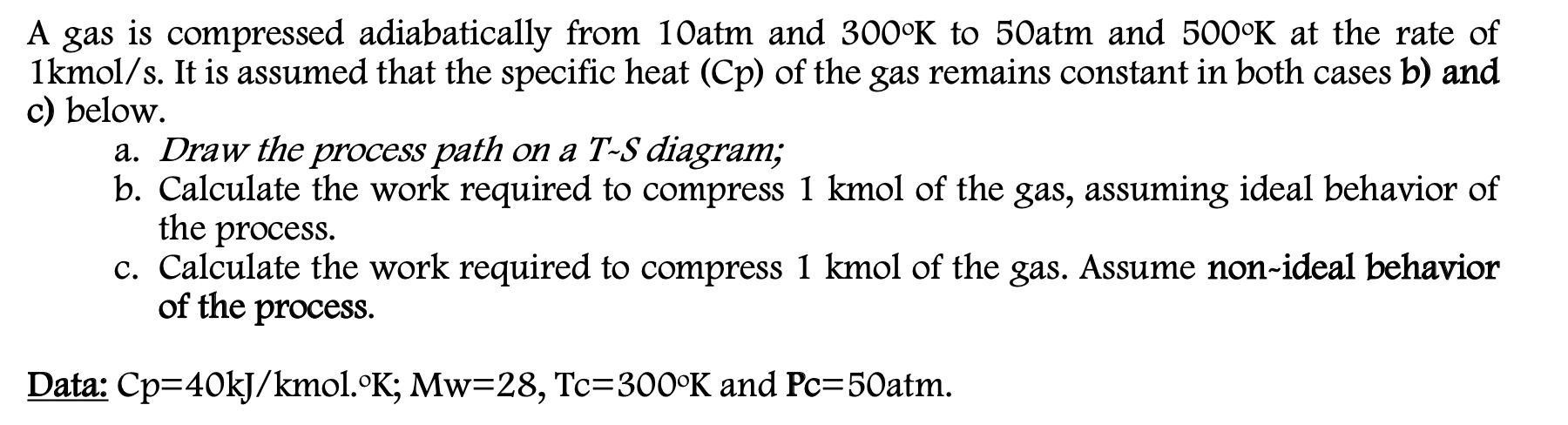
A gas is compressed adiabatically from 10atm and 3000K to 50atm and 5000K at the rate of 1kmol/s. It is assumed that the specific heat (Cp) of the gas remains constant in both cases b) and c) below. a. Draw the process path on a T-S diagram; b. Calculate the work required to compress 1 kmol of the gas, assuming ideal behavior of the process. c. Calculate the work required to compress 1 kmol of the gas. Assume non-ideal behavior of the process. Data: Cp=40kJ/kmol. K; Mw=28, Tc=300K and Pc=50atm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


