Question: answer all parts please a-c (7%) Problem 11: Suppose you want to raise the temperature of a mass m of ice from To 100 .C.
answer all parts please a-c
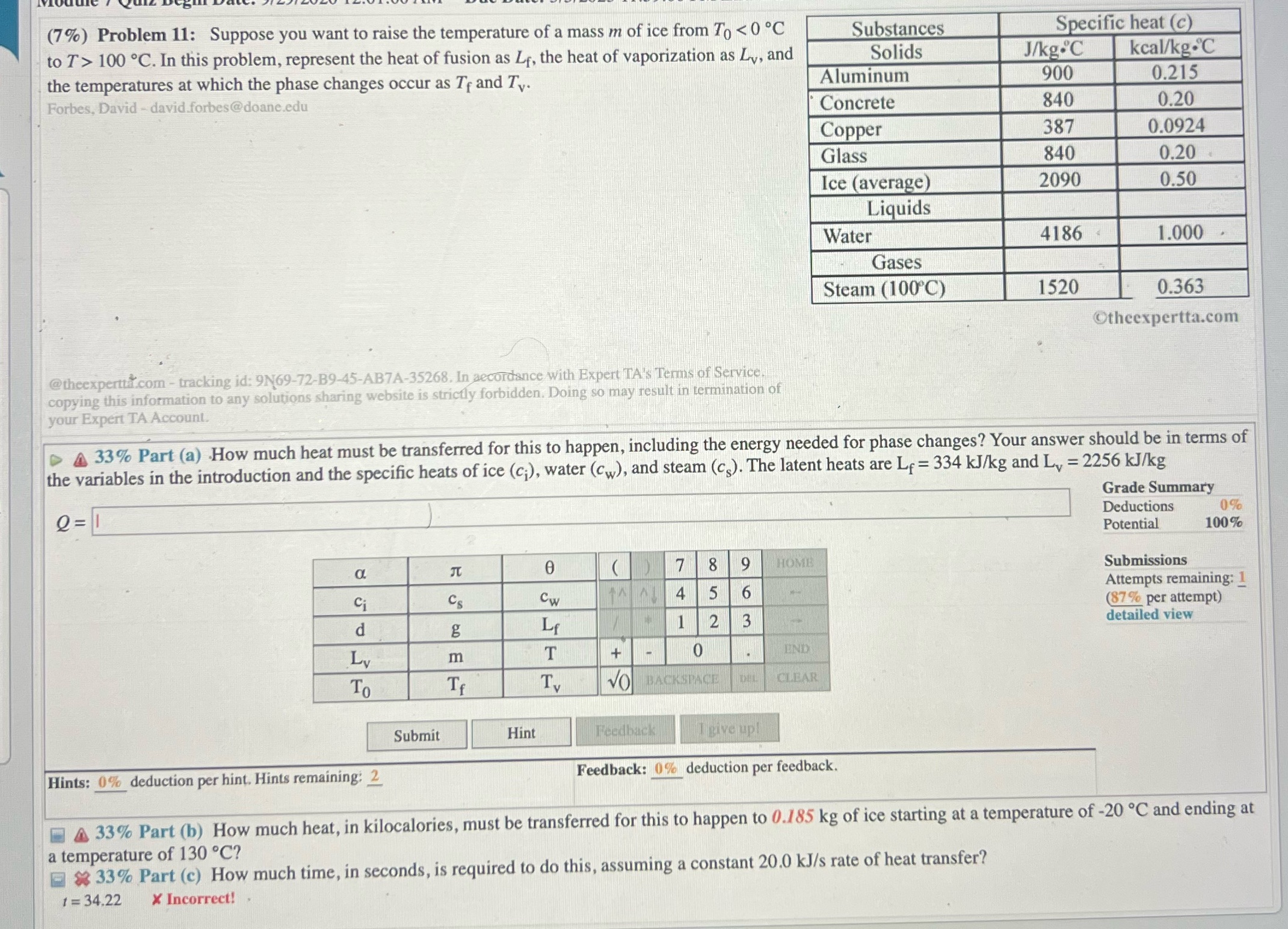
(7%) Problem 11: Suppose you want to raise the temperature of a mass m of ice from To 100 .C. In this problem, represent the heat of fusion as Ly, the heat of vaporization as Ly, and Solids J/kg.C kcal/kg.C the temperatures at which the phase changes occur as Ty and Tv. Aluminum 900 0.215 Forbes, David - david.forbes@doane.edu Concrete 840 0.20 Copper 387 0.0924 Glass 840 0.20 Ice (average) 2090 0.50 Liquids Water 4186 1.000 Gases Steam (100 C) 1520 0.363 theexpertta.com @theexperttal.com - tracking id: 9N69-72-B9-45-AB7A-35268. In accordance with Expert TA's Terms of Service. copying this information to any solutions sharing website is strictly forbidden. Doing so may result in termination of your Expert TA Account. 4 33% Part (a) How much heat must be transferred for this to happen, including the energy needed for phase changes? Your answer should be in terms of the variables in the introduction and the specific heats of ice (c;), water (Cw), and steam (C). The latent heats are Ly = 334 kJ/kg and Ly = 2256 kJ/kg Grade Summary Q=1 Deductions 0% Potential 100% 7 8 19 HOME Submissions Ci CS CW 4 5 6 Attempts remaining: 1 (87% per attempt) d g Lf 1 2 3 detailed view Ly m T + 0 END To If T. VO BACKSPACE CLEAR Submit Hint Feedback I give up! Hints: 0% deduction per hint. Hints remaining: 2 Feedback: 0% deduction per feedback. A 33% Part (b) How much heat, in kilocalories, must be transferred for this to happen to 0.185 kg of ice starting at a temperature of -20 .C and ending at a temperature of 130 .C? * 33% Part (c) How much time, in seconds, is required to do this, assuming a constant 20.0 kJ/s rate of heat transfer? 1 = 34.22 * Incorrect
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


