Question: answer all question AB+2Crn1, reversible A+2DE+3Frn 2 , irreversible N.B.1. if your answer is a fraction, please write it using a decimal point to two

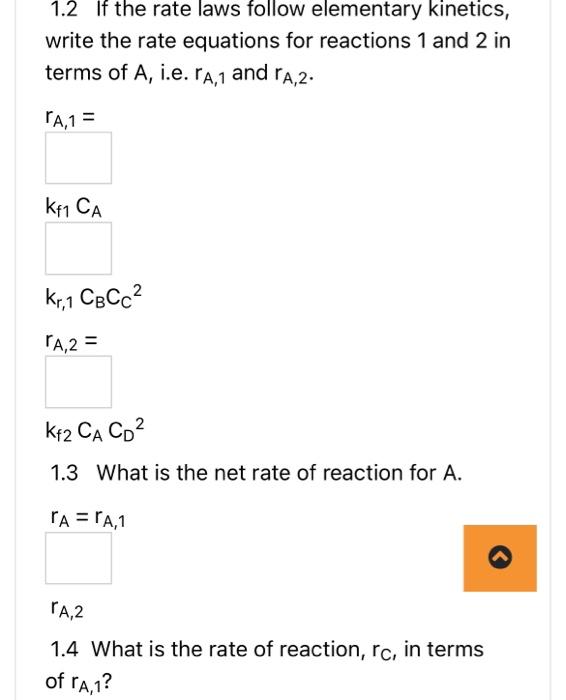

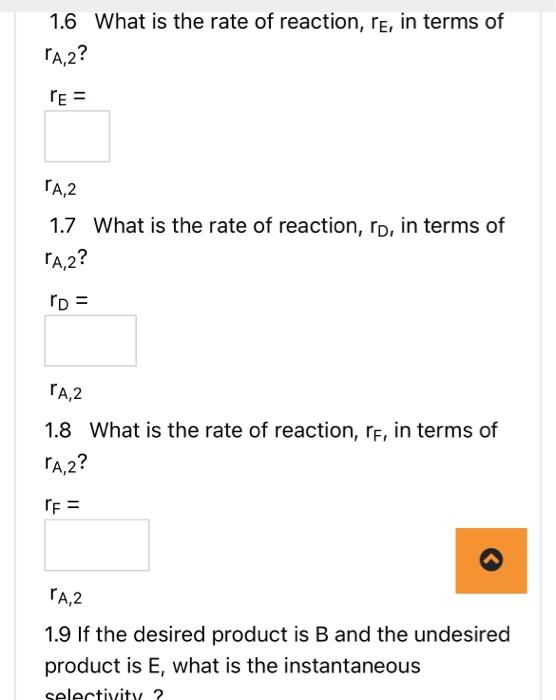
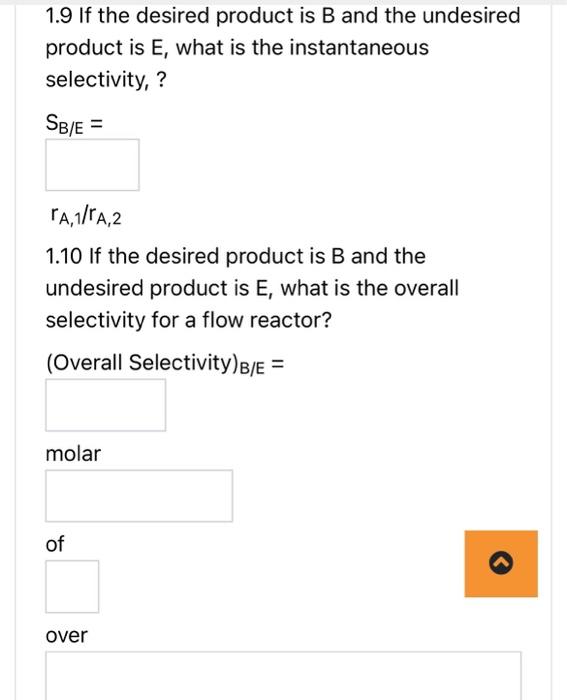
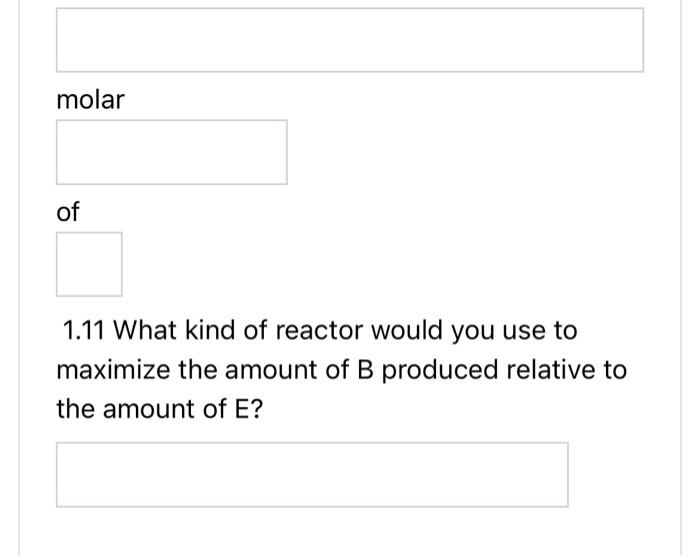
AB+2Crn1, reversible A+2DE+3Frn 2 , irreversible N.B.1. if your answer is a fraction, please write it using a decimal point to two decimal places. For example write 1/3 as 0.33 . N.B. 2 if your answer is + or - , please type +1 or 1. 1.1 Are these series, parallel or complex reactions? 1.2 If the rate laws follow elementary kinetics, write the rate equations for reactions 1 and 2 in terms of A, i.e. rA,1 and rA,2. rA,1= kf1CA kr,1CBCC2rA,2= kf2CACD2 1.3 What is the net rate of reaction for A. rA=rA,1 rA,2 1.4 What is the rate of reaction, rC, in terms of rA,1 ? 1.5 What is the rate of reaction, rB, in terms of rA,1? rB= rA,1 1.6 What is the rate of reaction, rE, in terms of rA,2? rE= 1.6 What is the rate of reaction, rE, in terms of rA,2? rE= rA,2 1.7 What is the rate of reaction, rD, in terms of rA,2? rD= rA,2 1.8 What is the rate of reaction, rF, in terms of rA,2 ? rF= rA,2 1.9 If the desired product is B and the undesired product is E, what is the instantaneous 1.9 If the desired product is B and the undesired product is E, what is the instantaneous selectivity,? SB/E= rA,1/rA,2 1.10 If the desired product is B and the undesired product is E, what is the overall selectivity for a flow reactor? (Overall Selectivity )B/E= molar 1.11 What kind of reactor would you use to maximize the amount of B produced relative to the amount of E ? AB+2Crn1, reversible A+2DE+3Frn 2 , irreversible N.B.1. if your answer is a fraction, please write it using a decimal point to two decimal places. For example write 1/3 as 0.33 . N.B. 2 if your answer is + or - , please type +1 or 1. 1.1 Are these series, parallel or complex reactions? 1.2 If the rate laws follow elementary kinetics, write the rate equations for reactions 1 and 2 in terms of A, i.e. rA,1 and rA,2. rA,1= kf1CA kr,1CBCC2rA,2= kf2CACD2 1.3 What is the net rate of reaction for A. rA=rA,1 rA,2 1.4 What is the rate of reaction, rC, in terms of rA,1 ? 1.5 What is the rate of reaction, rB, in terms of rA,1? rB= rA,1 1.6 What is the rate of reaction, rE, in terms of rA,2? rE= 1.6 What is the rate of reaction, rE, in terms of rA,2? rE= rA,2 1.7 What is the rate of reaction, rD, in terms of rA,2? rD= rA,2 1.8 What is the rate of reaction, rF, in terms of rA,2 ? rF= rA,2 1.9 If the desired product is B and the undesired product is E, what is the instantaneous 1.9 If the desired product is B and the undesired product is E, what is the instantaneous selectivity,? SB/E= rA,1/rA,2 1.10 If the desired product is B and the undesired product is E, what is the overall selectivity for a flow reactor? (Overall Selectivity )B/E= molar 1.11 What kind of reactor would you use to maximize the amount of B produced relative to the amount of E
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


