Question: Please answer with an explanation. Question 10 10 pts A forensic scientist determines the percent composition of a hydrocarbon to be 65% carbon, 14% hydrogen
 Please answer with an explanation.
Please answer with an explanation.
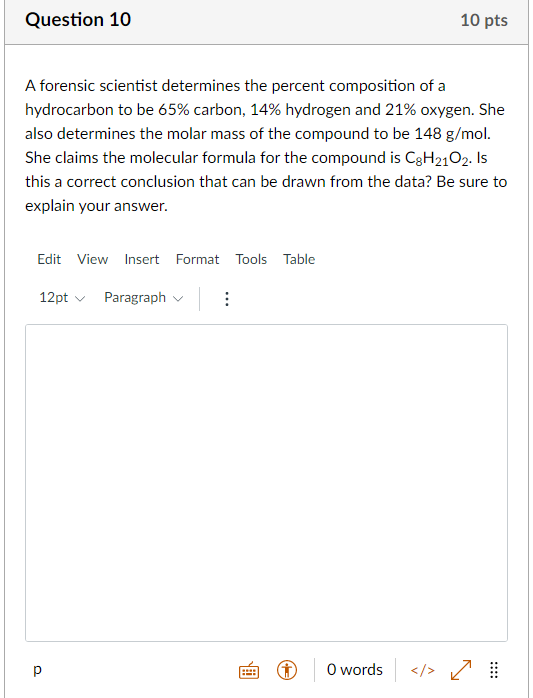
Question 10 10 pts A forensic scientist determines the percent composition of a hydrocarbon to be 65% carbon, 14% hydrogen and 21% oxygen. She also determines the molar mass of the compound to be 148 g/mol. She claims the molecular formula for the compound is C3H2102. Is this a correct conclusion that can be drawn from the data? Be sure to explain your answer. Edit View Insert Format Tools Table 12pt Paragraph O words > t
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


