Question: answer all steps please In a study of the decomposition of nitrosyl bromide at 10C NOBrNO+1/2Br2 the following data were obtained: Hint: It is not
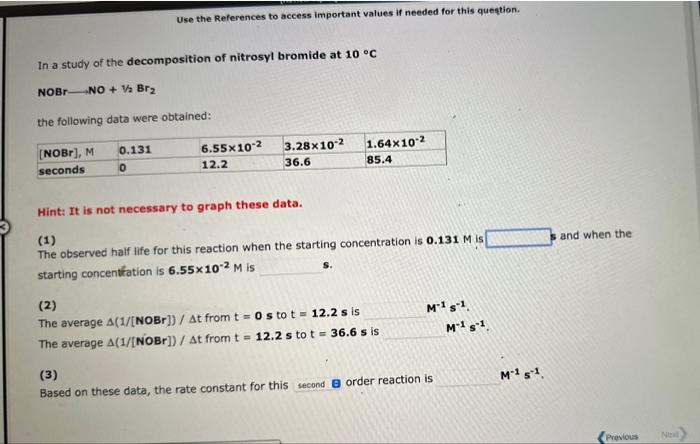
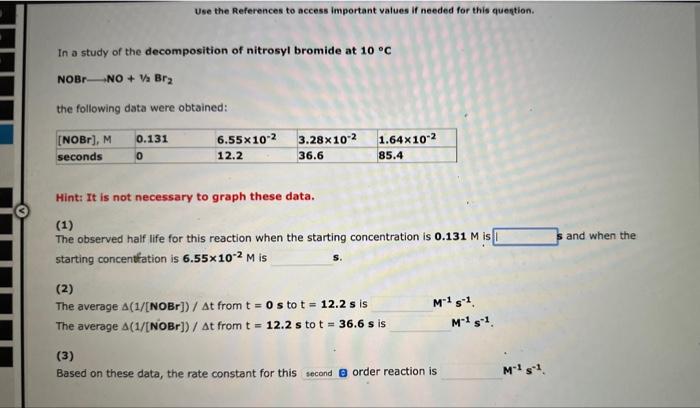
In a study of the decomposition of nitrosyl bromide at 10C NOBrNO+1/2Br2 the following data were obtained: Hint: It is not necessary to graph these data. (1) The observed half life for this reaction when the starting concentration is 0.131M is i and when the starting concentration is 6.55102M is (2) The average (1/[NOBr])/t from t=0s to t=12.2s is M1s1. The average (1/[NOBr])/t from t=12.2s to t=36.6s is M1s1. (3) Based on these data, the rate constant for this order reaction is M1s1. Use the References to access important values if needed for this question. In a study of the decomposition of nitrosyl bromide at 10C NOBrNO+V2Br2 the following data were obtained: Hint: It is not necessary to graph these data. (1) The observed half life for this reaction when the starting concentration is 0.131M is and when the starting concentfation is 6.55102M is (2) The average (1/[NOBr])/t from t=0s to t=12.2s is M1s1, The average (1/[NOBr])/t from t=12.2s to t=36.6s is M1s1. (3) Based on these data, the rate constant for this order reaction is M1s1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


