Question: Please explain fully. The following data are for the rearrangement of cyclopropane to propene at 500 C. (CH2)3 CH3CH=CH2 [(CH2)3 ), M 0.170 time, min
Please explain fully.
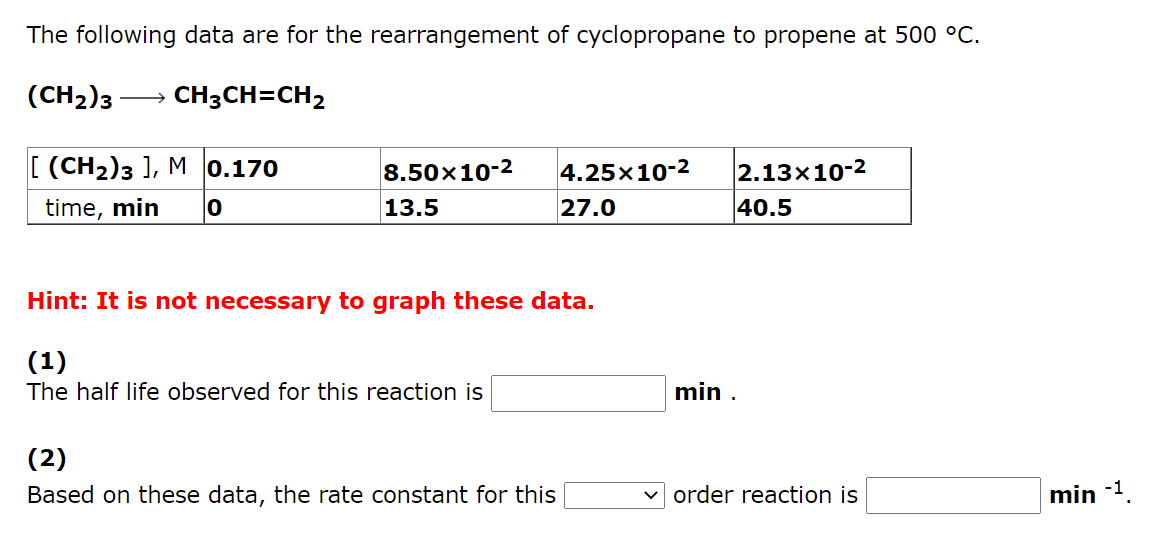
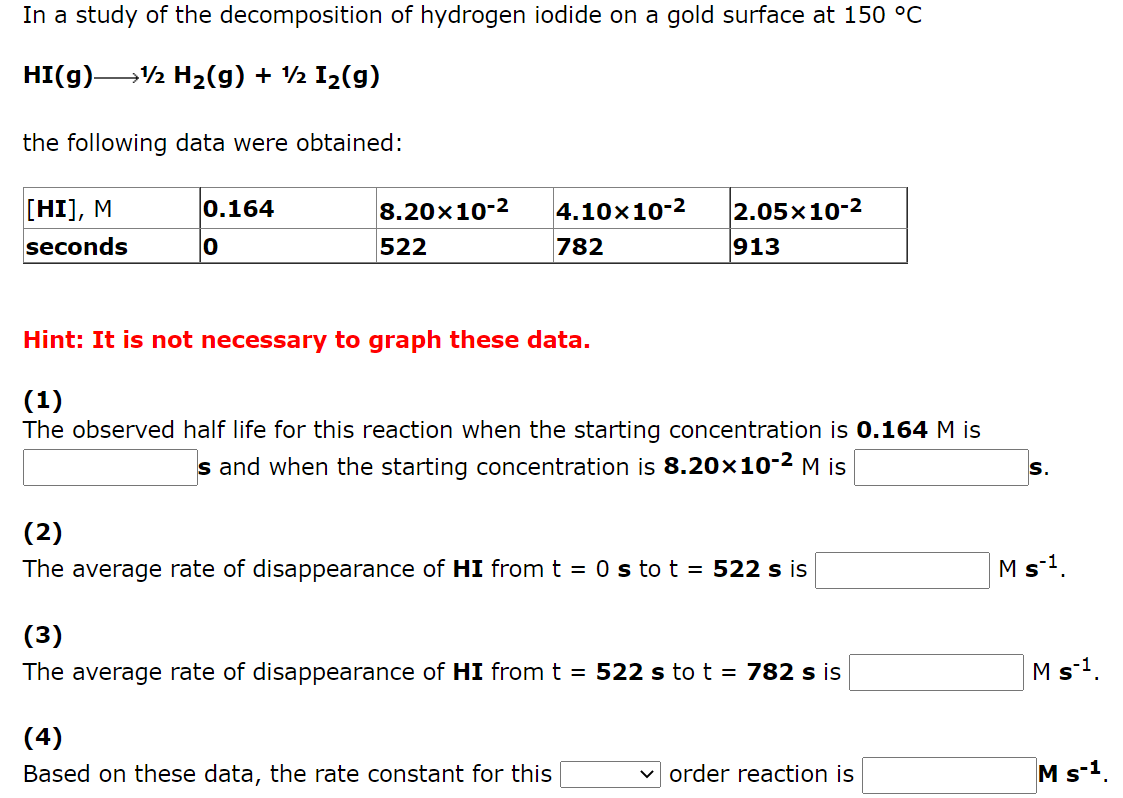
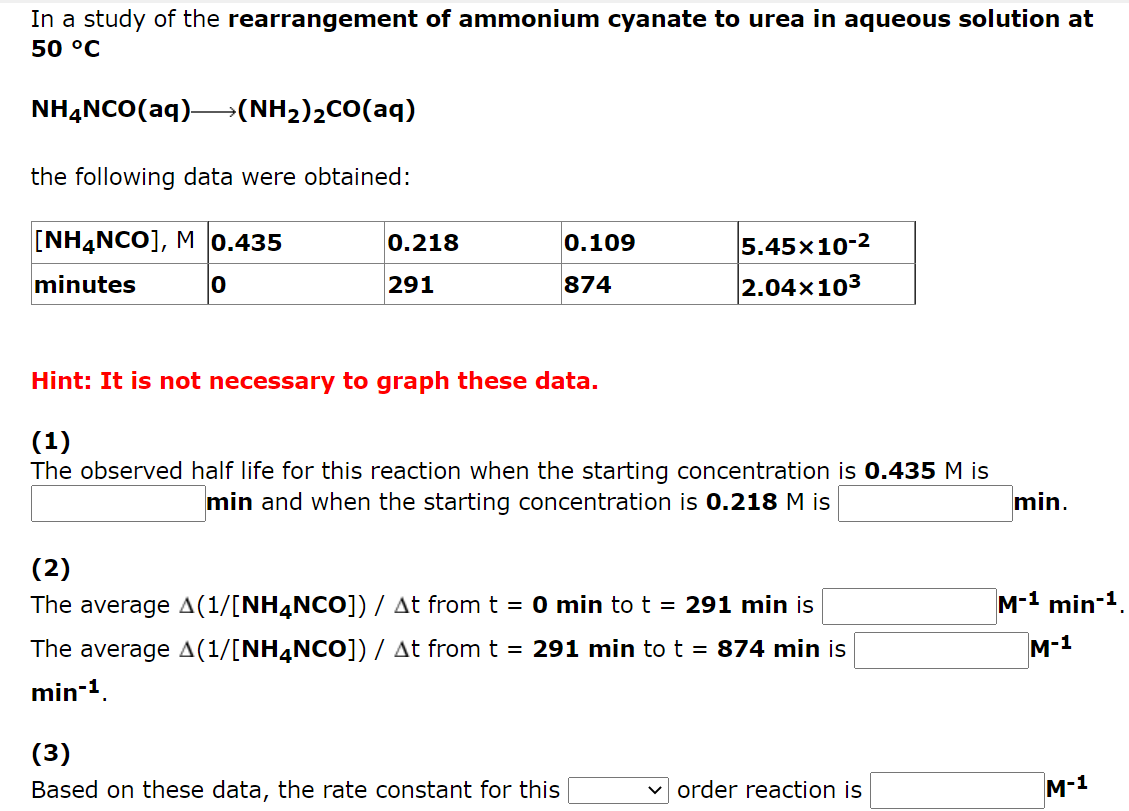
The following data are for the rearrangement of cyclopropane to propene at 500 C. (CH2)3 CH3CH=CH2 [(CH2)3 ), M 0.170 time, min 8.50x10-2 13.5 4.25x10-2 27.0 2.13x10-2 40.5 0 Hint: It is not necessary to graph these data. (1) The half life observed for this reaction is min. (2) Based on these data, the rate constant for this v order reaction is min -1 In a study of the decomposition of hydrogen iodide on a gold surface at 150 C HI(g)1/2 H2(g) + 1/2 I2(g) the following data were obtained: 0.164 [HI], M. seconds 8.20x10-2 522 4.10x10-2 782 2.05x10-2 913 0 Hint: It is not necessary to graph these data. (1) The observed half life for this reaction when the starting concentration is 0.164 M is s and when the starting concentration is 8.20x10-2 M is (2) The average rate of disappearance of HI from t = 0 s to t = 522 s is Ms1 (3 ) The average rate of disappearance of HI from t = 522 s to t = 782 s is Ms-1. (4) Based on these data, the rate constant for this v order reaction is Ms-1 In a study of the rearrangement of ammonium cyanate to urea in aqueous solution at 50 C NH4NCO(aq)->(NH2)2CO(aq) the following data were obtained: [NH4NCO], M 0.435 0.218 0.109 5.45x 10-2 2.04x 103 minutes O 291 874 Hint: It is not necessary to graph these data. (1) The observed half life for this reaction when the starting concentration is 0.435 M is min and when the starting concentration is 0.218 M is min. M-1 min-1 (2) The average A(1/(NH4NCO]) / At from t = 0 min to t = 291 min is The average A(1/[NH4NCO]) / At from t = 291 min to t = 874 min is min-1 M-1 (3) Based on these data, the rate constant for this order reaction is M-1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


