Question: ANSWER ALLL The compound sucrose is also known as table sugar. It has the formula C12H22O11. How many moles of sucrose are contained in a


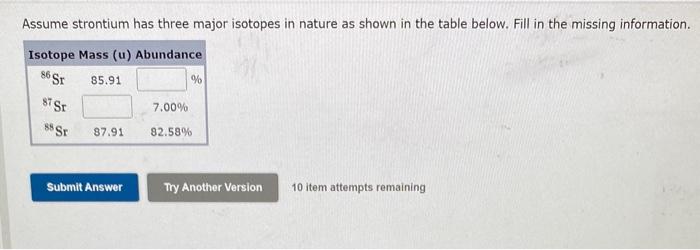
The compound sucrose is also known as table sugar. It has the formula C12H22O11. How many moles of sucrose are contained in a sample with a mass of 181.6g ? HOW DO WE GET THERE? How many moles of sucrose are contained in a sample with a mass of 181.6g ? The molar mass of sucrose is 342.3g/mol. mol ( Next) (3 of 3) How many water (H2O) molecules are in a block of ice that has a mass of 56.7g ? How many atoms of hydrogen are present in this block? HOW DO WE GET THERE? How many moles are present in the 56.7g block of ice? mol ( Next) (3 of 5) Assume strontium has three major isotopes in nature as shown in the table below. Fill in the missing information. 10 item attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


