Question: answer as a chemical formula please ! MISSED THIS? Read Section 4.4 (Pages 149 - 155): Watch KCV 4.4. IWE 4.6. 2.5molNa and 1molBr2 Find
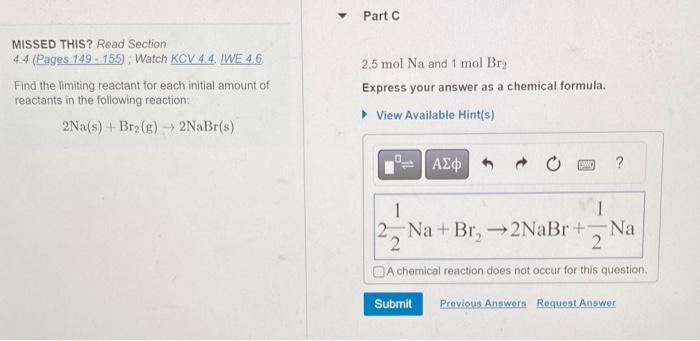
MISSED THIS? Read Section 4.4 (Pages 149 - 155): Watch KCV 4.4. IWE 4.6. 2.5molNa and 1molBr2 Find the limiting reactant for each initial amount of Express your answer as a chemical formula. reactants in the following reaction: 2Na(s)+Br2(g)2NaBr(s)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


