Question: MISSED THIS? Read Section 4.4 (Pages 149 - 155) ; Watch KCV 4.4, IWE 4.6. For the reaction Ti(s)+2F2(g)TiF4(s) compute the theoretical yield of the

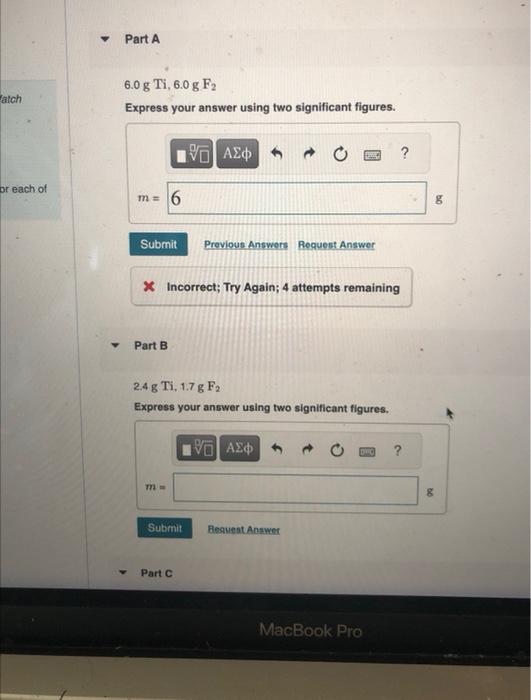
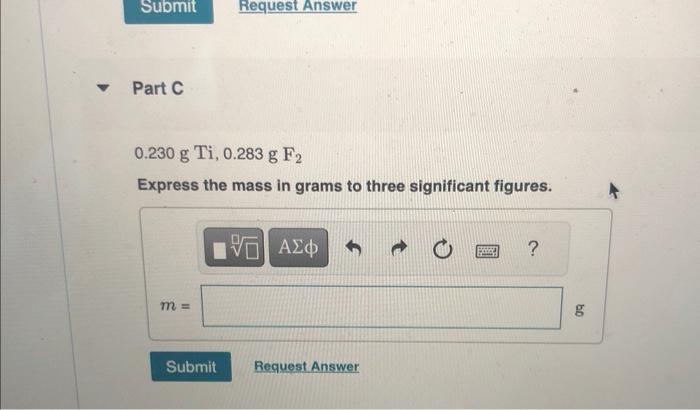
MISSED THIS? Read Section 4.4 (Pages 149 - 155) ; Watch KCV 4.4, IWE 4.6. For the reaction Ti(s)+2F2(g)TiF4(s) compute the theoretical yield of the product (in grams) for each of the following initial amounts of reactants. 6.0gTi,6.0gF2 Express your answer using two significant figures. X Incorrect; Try Again; 4 attempts remaining Part B 2.4gTi,1.7gF Express your answer using two significant flgures. 0.230gTi,0.283gFF2 Express the mass in grams to three significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


