Question: ANSWER BOTH PARTS WITH CORRECT SIG FIGS AND I WILL LEAVE A LIKE/GOOD REVIEW!! pls Post-Lab 1 [References] Use the References to access important values
![LIKE/GOOD REVIEW!! pls Post-Lab 1 [References] Use the References to access important](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f909da25a88_01766f909d99cb05.jpg) ANSWER BOTH PARTS WITH CORRECT SIG FIGS AND I WILL LEAVE A LIKE/GOOD REVIEW!! pls
ANSWER BOTH PARTS WITH CORRECT SIG FIGS AND I WILL LEAVE A LIKE/GOOD REVIEW!! pls
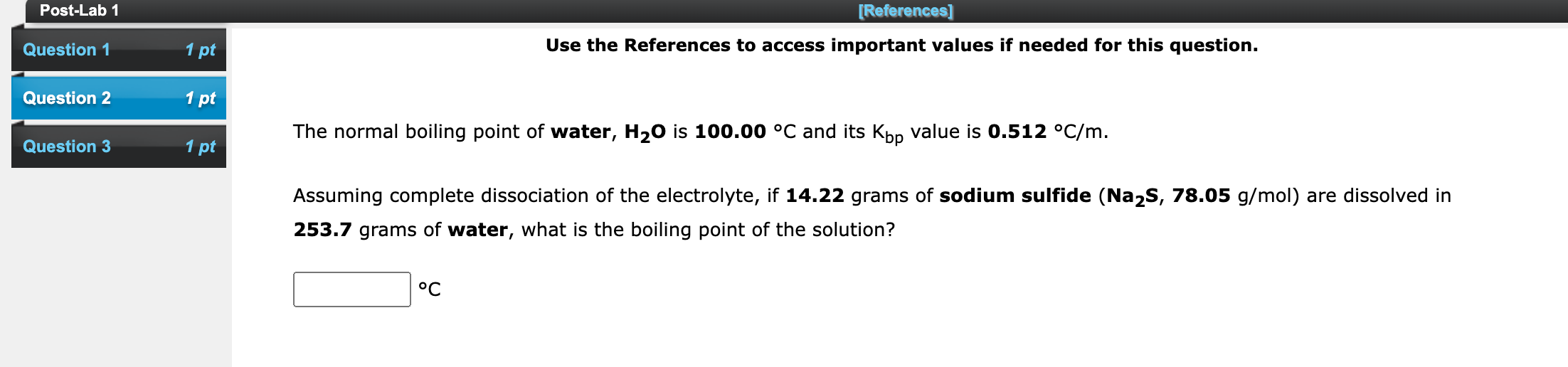
Post-Lab 1 [References] Use the References to access important values if needed for this question. Question 1 1 pt Question 2 1 pt The normal boiling point of water, H20 is 100.00 C and its Kbp value is 0.512 C/m. Question 3 1 pt Assuming complete dissociation of the electrolyte, if 14.22 grams of sodium sulfide (Na2S, 78.05 g/mol) are dissolved in 253.7 grams of water, what is the boiling point of the solution? C Post-Lab 1 [References] Use the References to access important values if needed for this question. Question 1 1 pt Question 2 1 pt Question 3 1 pt The normal boiling point of benzene (C6H6) is 80.1 C. If 42.00 grams of the nonvolatile nonelectrolyte 5-bromo-2-naphthoic acid (C11H_BrO2), are dissolved in 278.4 grams of benzene, what is the boiling point of the resulting solution? Kbp for benzene is 2.53 C/m. oC
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


