Question: Use the References to access important values if needed for this question. Write the net ionic equation for the following molecular equation. CoCl2(aq) + K2CO3(aq)
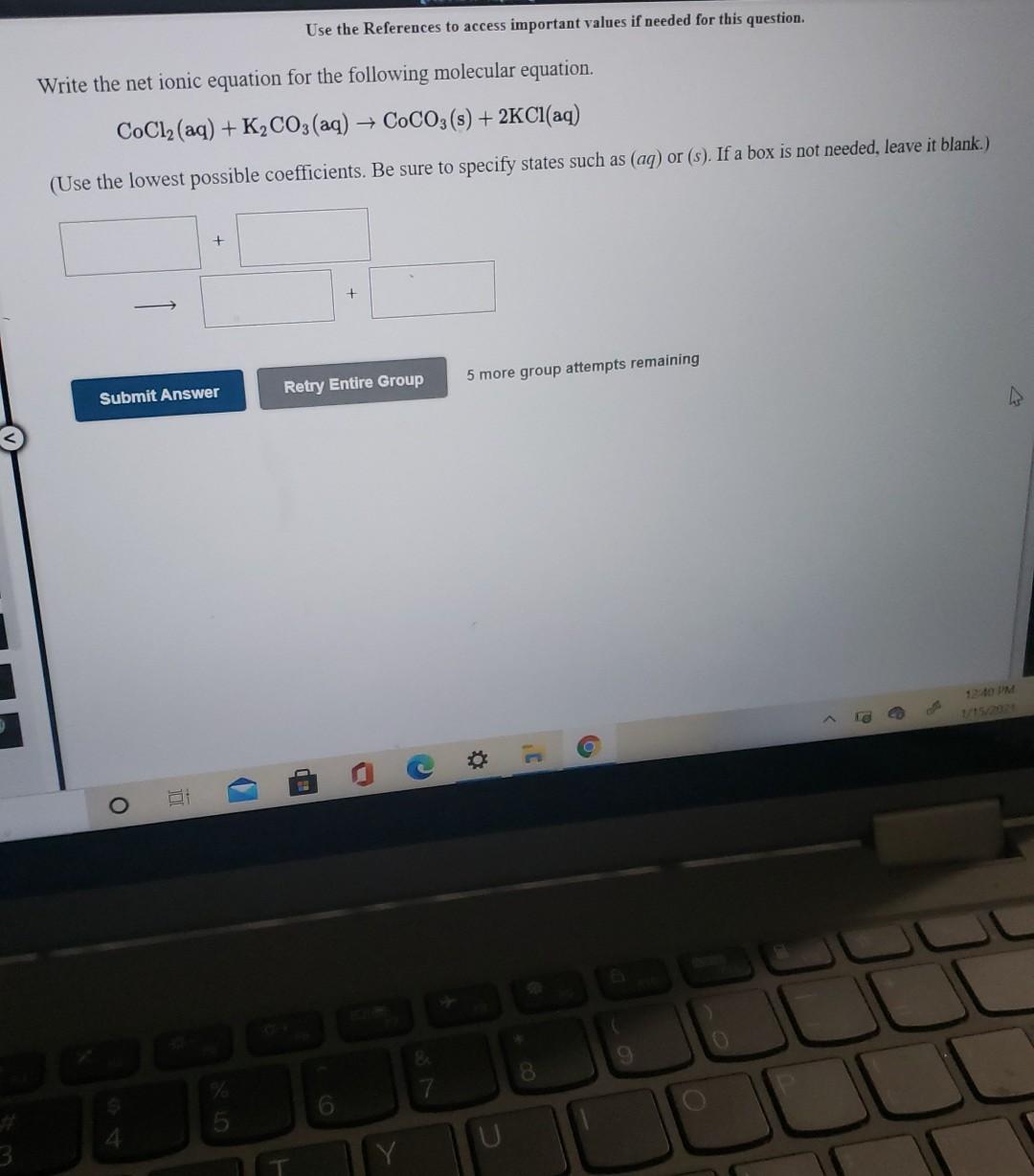
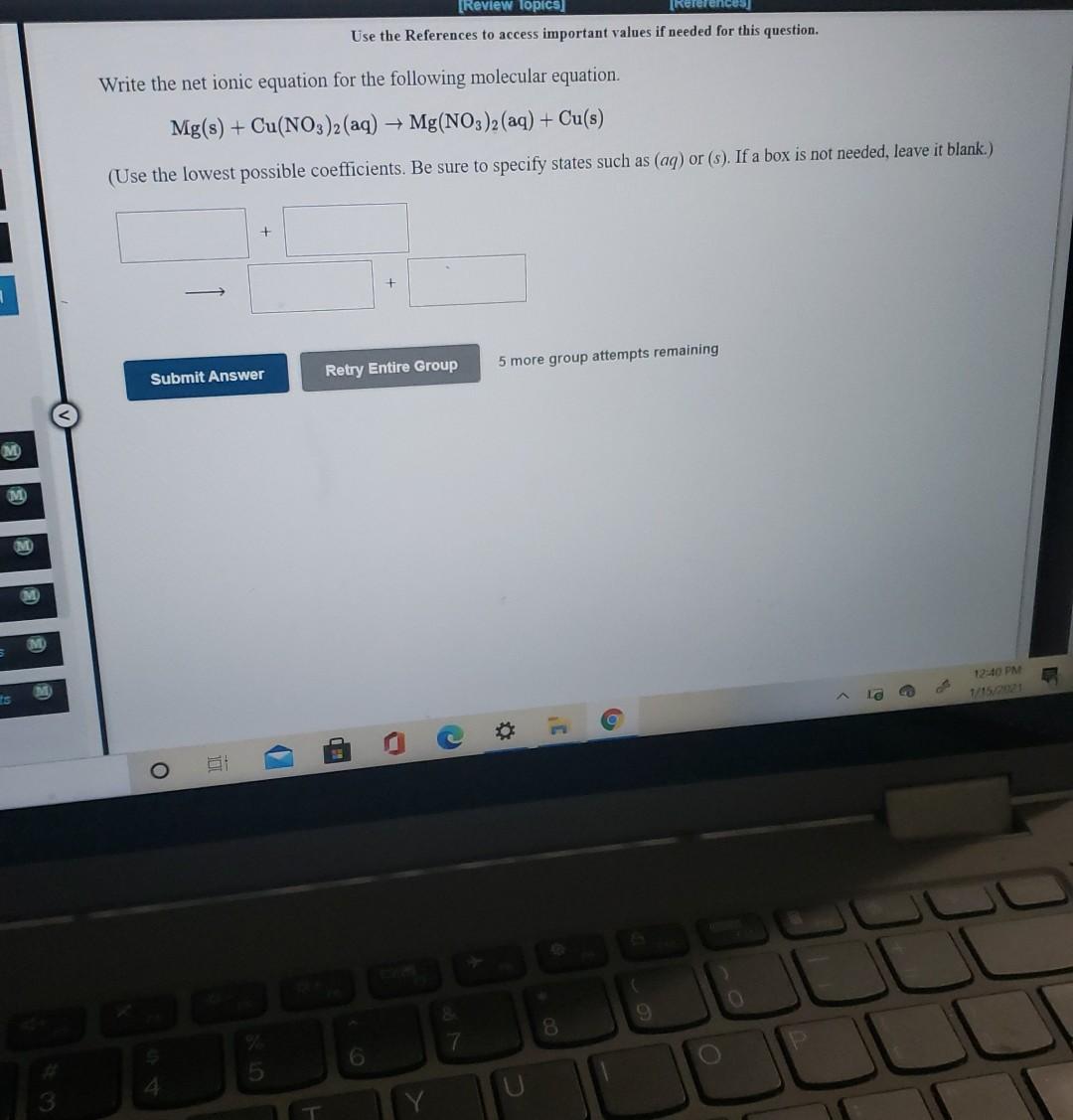
Use the References to access important values if needed for this question. Write the net ionic equation for the following molecular equation. CoCl2(aq) + K2CO3(aq) + COCO3(s) + 2Cl(aq) (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) + 5 more group attempts remaining Retry Entire Group Submit Answer g O 9 8 6 [Review Topics] (References Use the References to access important values if needed for this question. Write the net ionic equation for the following molecular equation. Mg(s) + Cu(NO3)2(aq) + Mg(NO3)2 (aq) + Cu(s) (Use the lowest possible coefficients. Be sure to specify states such as (aq) or (s). If a box is not needed, leave it blank.) + + Submit Answer Retry Entire Group 5 more group attempts remaining M 12:40 PM La is C 9 8. 6
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


