Question: Answer both parts with full steps to get 100% feedback!!! Q: 3 m 3 In a tank : Methane Volume of Pressure 300 k .
Answer both parts with full steps to get 100% feedback!!!
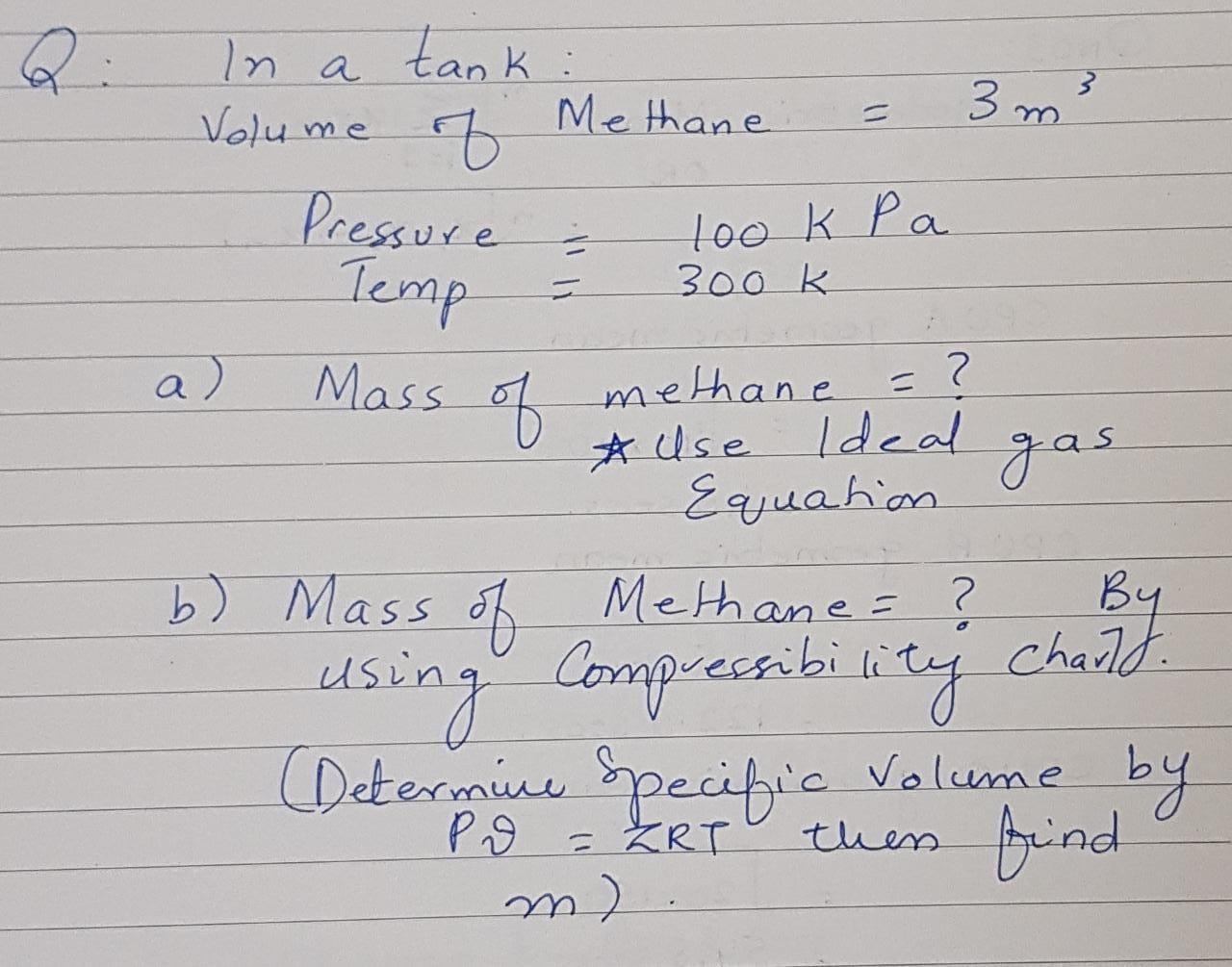
Q: 3 m 3 In a tank : Methane Volume of Pressure 300 k . look pa Temp a) Mass of = methane = ? ? * Use Ideal Equation gas ? By b) Mass of Methane = ? b . using (Determine specific Volume by then find m) Compressibility chaild PO = ZRT
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


