Question: Answer both question 1 With proper sig fig** 1. Fill in the blanks in the table for aqueous solutions of the compounds shown. The density
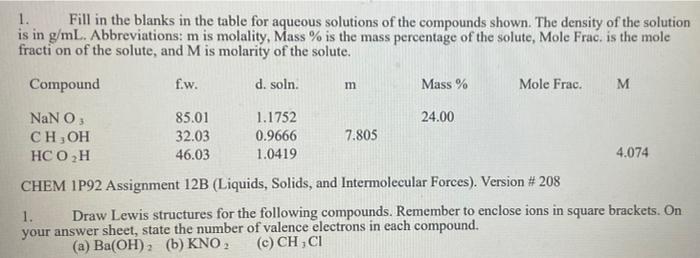
1. Fill in the blanks in the table for aqueous solutions of the compounds shown. The density of the solution is in g/mL. Abbreviations: m is molality, Mass % is the mass percentage of the solute, Mole Frac. is the mole fraction of the solute, and M is molarity of the solute. Compound fw. d. soln. Mass % Mole Frac. M m NaN O 85.01 1.1752 24.00 CH, OH 32.03 0.9666 7.805 HC 0 H 46.03 1.0419 4.074 CHEM 1P92 Assignment 12B (Liquids, Solids, and Intermolecular Forces). Version # 208 1. Draw Lewis structures for the following compounds. Remember to enclose ions in square brackets. On your answer sheet, state the number of valence electrons in each compound. (a) Ba(OH)2(b) KNO, (c) CHCI
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


