Question: answer clearly and quickly pleaseeeee 01) (75 Pts The liquid-phase reaction (A+B+C) takes place isothermally at a steady state. The volumetric flow rate is 10
answer clearly and quickly pleaseeeee
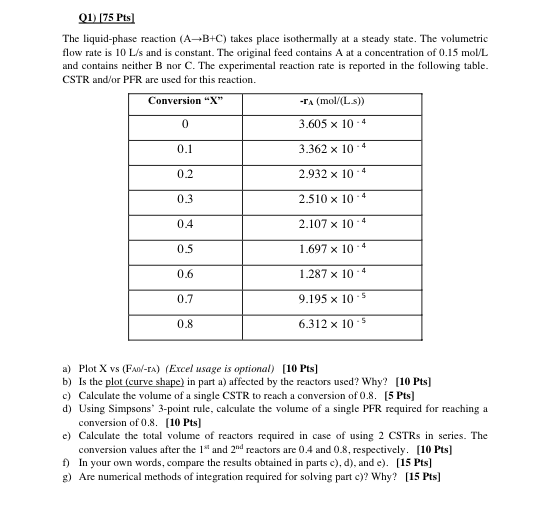
01) (75 Pts The liquid-phase reaction (A+B+C) takes place isothermally at a steady state. The volumetric flow rate is 10 L/s and is constant. The original feed contains A at a concentration of 0.15 mol/L and contains neither B nor C. The experimental reaction rate is reported in the following table. CSTR and/or PFR are used for this reaction. Conversion "X" -TA (mol/(Ls)) 0 3.605 x 10-4 0.1 3.362 x 10-4 0.2 2.932 x 10-4 2.510 x 10+ 0.3 4 0.4 2.107 x 10-4 0.5 1.697 x 10 0.6 1.287 x 10.4 0.7 9.195 X 10.5 0.8 6.312 x 10-5 a) Plot X vs (Faol-ra) (Excel usage is optional) [10 Pts] b) Is the plot (curve shape) in part a) affected by the reactors used? Why? (10 Pts] c) Calculate the volume of a single CSTR to reach a conversion of 0.8. [5 Pts] d) Using Simpsons" 3-point rule, calculate the volume of a single PFR required for reaching a conversion of 0.8. [10 Pts] e) Calculate the total volume of reactors required in case of using 2 CSTRs in series. The conversion values after the 1 and 200 reactors are 0.4 and 0.8, respectively. [10 Pts] f) In your own words, compare the results obtained in parts c), d), and e). [15 Pts] g) Are numerical methods of integration required for solving part e)? Why? (15 Pts]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


