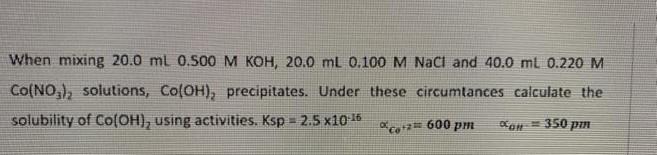
Question: answer correctly completely thanks When mixing 20.0 ml 0.500 M KOH, 20.0 mL 0.100 M Naci and 40.0 ml 0.220 M CO(NOx), solutions, ColOH), precipitates.
answer correctly completely thanks
When mixing 20.0 ml 0.500 M KOH, 20.0 mL 0.100 M Naci and 40.0 ml 0.220 M CO(NOx), solutions, ColOH), precipitates. Under these circumtances calculate the solubility of Co(OH), using activities. Ksp = 2.5 x10-16 Co+*+ 600 PM dan 350 pon
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


