Question: 5.35 gms of NH4Clis added to 1 lit of 0.4 M NH4OHsolution. Find the [OH Jof the resulting solution assuming no change in volume
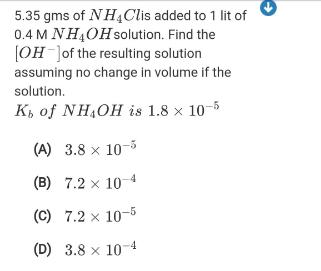
5.35 gms of NH4Clis added to 1 lit of 0.4 M NH4OHsolution. Find the [OH Jof the resulting solution assuming no change in volume if the solution. K, of NH,OH is 1.8 x 10-5 (A) 3.8 x 10-5 (B) 7.2 x 10-4 (C) 7.2 x 10-5 (D) 3.8 x 104
Step by Step Solution
3.51 Rating (161 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


