Question: answer for a and b with solution pls For Practice 15.1 - Enhanced - with Feedback Predict the rate of change in the concentration of
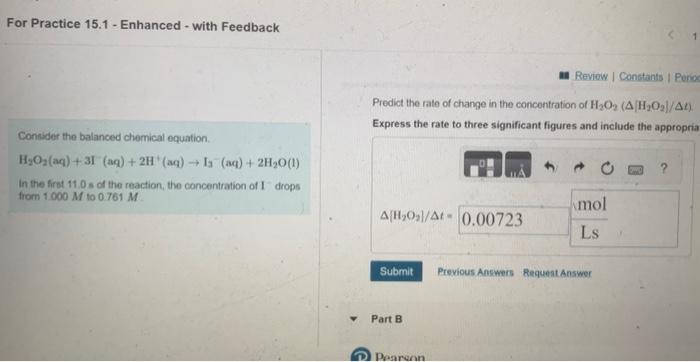
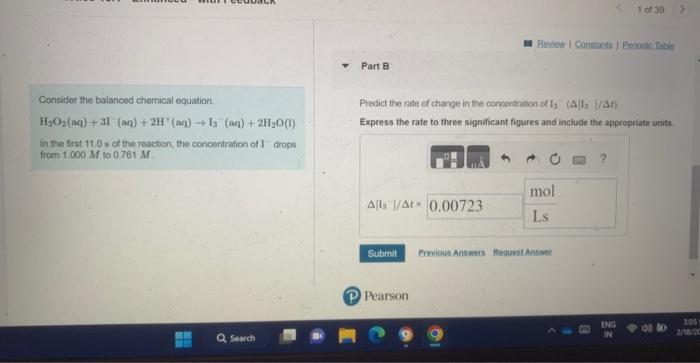
For Practice 15.1 - Enhanced - with Feedback Predict the rate of change in the concentration of H2O2([H2O2]/). Express the rate to three significant figures and include the appropria Connider the balanced chemical equation. H2O2(aq)+3I(aq)+2H(aq)I3(aq)+2H2O(1) In the first 11.0 s of the reaction, the concentration of I drops from 1.000M to 0.761M. Consider the balanced chemical equation. Predict the rate of change in the concentration of I3 (A) I3//t) H2O2(aq)+3I(aq)+2H(aq)I3(aq)+2H2O(l) Express the rate to three significant figures and include the appropriate unity. In the first 11.9 s of the reaction, the concentration of I drops from 1.000M to 0.761M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


