Question: answer is given, please help with the solition (: Na(s)+H2O(l)NaOH(aq)+H2(g) A sample of sodium metal is reacted with excess water and the resulting hydrogen gas

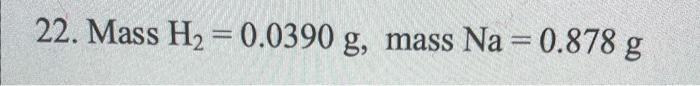
Na(s)+H2O(l)NaOH(aq)+H2(g) A sample of sodium metal is reacted with excess water and the resulting hydrogen gas fills a volume of 0.490L, collected above the water. The temperature of the gas is 298K and the total pressure is 758mmHg. Determine the mass of H2 produced and the mass of Na that reacted. 22. Mass H2=0.0390g, mass Na=0.878g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


