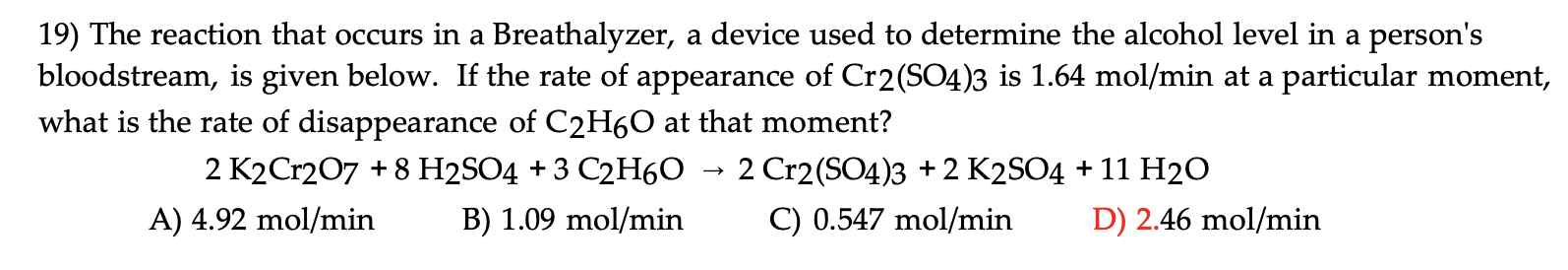
Question: Answer is in red please break it down 19) The reaction that occurs in a Breathalyzer, a device used to determine the alcohol level in
 Answer is in red please break it down
Answer is in red please break it down
19) The reaction that occurs in a Breathalyzer, a device used to determine the alcohol level in a person's bloodstream, is given below. If the rate of appearance of Cr2(SO4)3 is 1.64mol/min at a particular moment, what is the rate of disappearance of C2H6O at that moment? 2K2Cr2O7+8H2SO4+3C2H6O2Cr2(SO4)3+2K2SO4+11H2O A) 4.92mol/min B) 1.09mol/min C) 0.547mol/min D) 2.46mol/min
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


