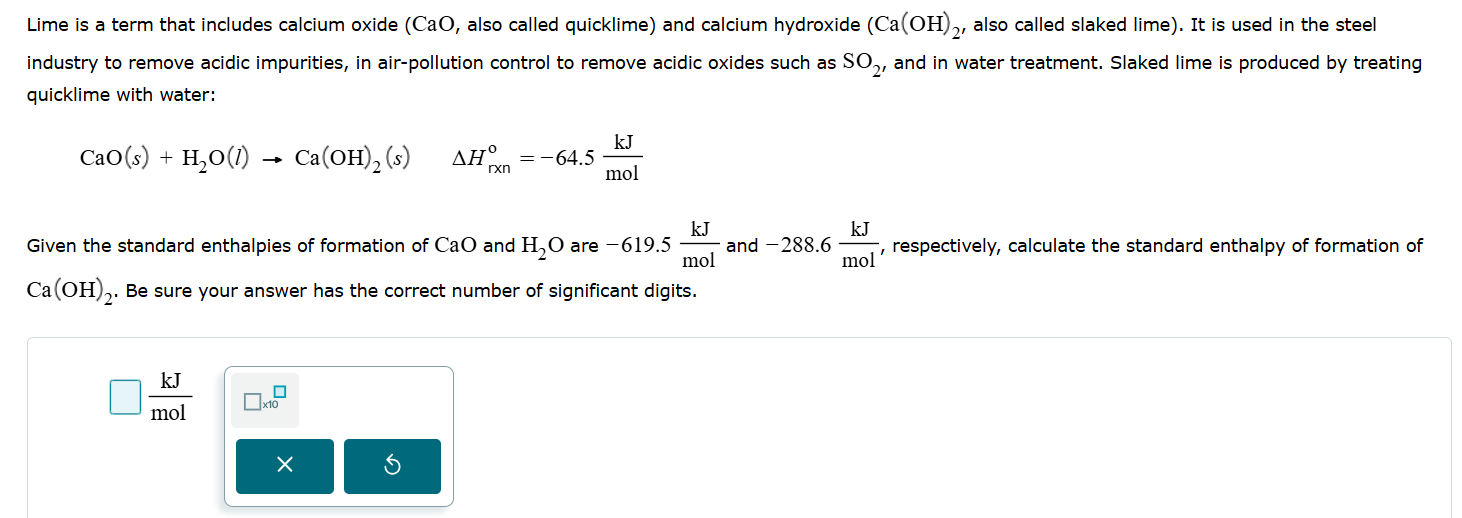
Question: answer Lime is a term that includes calcium oxide (CaO, also called quicklime) and calcium hydroxide (Ca(OH),, also called slaked lime). It is used in
answer
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


