Question: Lime is a term that includes calcium oxide (CaO, also called quicklime) and calcium hydroxide [Ca(OH)2, also called slaked lime]. It is used in
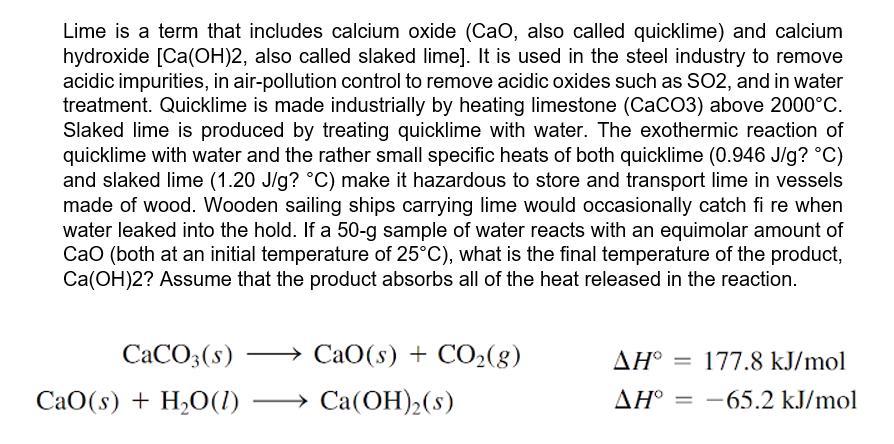
Lime is a term that includes calcium oxide (CaO, also called quicklime) and calcium hydroxide [Ca(OH)2, also called slaked lime]. It is used in the steel industry to remove acidic impurities, in air-pollution control to remove acidic oxides such as SO2, and in water treatment. Quicklime is made industrially by heating limestone (CaCO3) above 2000C. Slaked lime is produced by treating quicklime with water. The exothermic reaction of quicklime with water and the rather small specific heats of both quicklime (0.946 J/g? C) and slaked lime (1.20 J/g? C) make it hazardous to store and transport lime in vessels made of wood. Wooden sailing ships carrying lime would occasionally catch fi re when water leaked into the hold. If a 50-g sample of water reacts with an equimolar amount of Cao (both at an initial temperature of 25C), what is the final temperature of the product, Ca(OH)2? Assume that the product absorbs all of the heat released in the reaction. CACO3(s) CaO(s) + CO2(g) = 177.8 kJ/mol CaO(s) + H2O(1) Ca(OH)2(s) AH = -65.2 kJ/mol
Step by Step Solution
3.33 Rating (150 Votes )
There are 3 Steps involved in it
Given CaOH2OCaOH2H652KJmol Specific heat ofCaOH212JgmC To calculate the amount of heat released ... View full answer

Get step-by-step solutions from verified subject matter experts


