Question: answer need as soon as possible please (1) Why don't we observe the transition from n2 n for the hydrogen atom in the line spectrum?
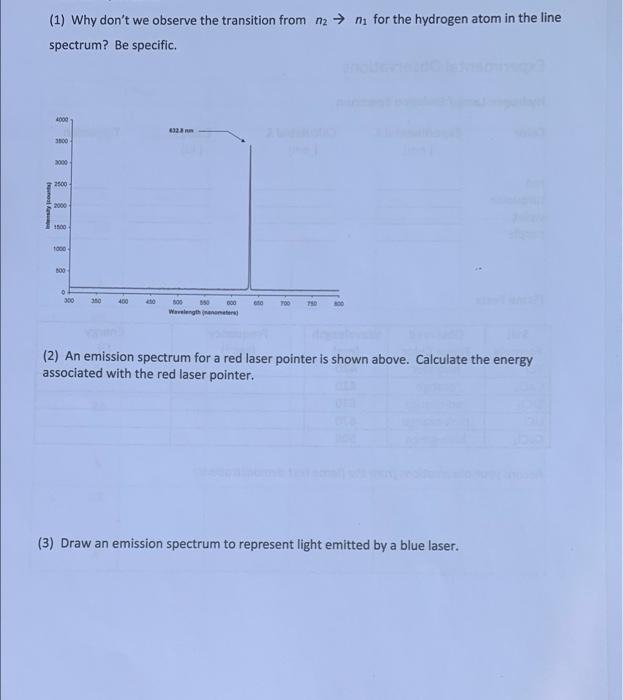
(1) Why don't we observe the transition from n2 n for the hydrogen atom in the line spectrum? Be specific 4000 3600 000 2800 2000 1800 1000 00 300 30 400 000 350 Wervelength (2) An emission spectrum for a red laser pointer is shown above. Calculate the energy associated with the red laser pointer. (3) Draw an emission spectrum to represent light emitted by a blue laser
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


