Question: answer number 2 please The conversion of cyclopropane to propene follows first-order kinetics. At 773K, the rate constant, k is 6.7104s1 and the initial concentration
answer number 2 please 

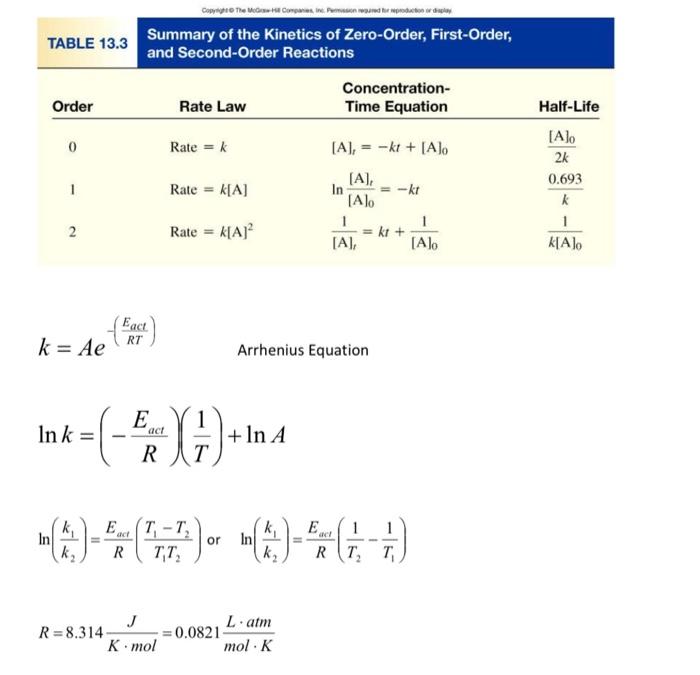
The conversion of cyclopropane to propene follows first-order kinetics. At 773K, the rate constant, k is 6.7104s1 and the initial concentration of cyclopropane is 0.100M. Calculate the concentration of cyclopropane after 200. s. [3 points] k=Ae(RTEact) Arrhenius Equation lnk=(REact)(T1)+lnA ln(k2k1)=REact(T1T2T1T2) or ln(k2k1)=REact(T21T11) R=8.314KmolJ=0.0821molKLatm
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


