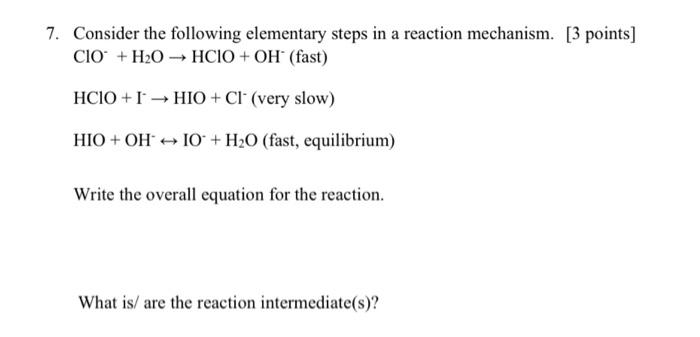
Question: answer number 7 please 7. Consider the following elementary steps in a reaction mechanism. [ 3 points] ClO+H2OHClO+OH(fast) HClO+IHIO+Cl(very slow) HIO+OHIO+H2O (fast, equilibrium) Write the
![a reaction mechanism. [ 3 points] ClO+H2OHClO+OH(fast) HClO+IHIO+Cl(very slow) HIO+OHIO+H2O (fast, equilibrium)](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f8e45a2f259_41766f8e459c2082.jpg)
7. Consider the following elementary steps in a reaction mechanism. [ 3 points] ClO+H2OHClO+OH(fast) HClO+IHIO+Cl(very slow) HIO+OHIO+H2O (fast, equilibrium) Write the overall equation for the reaction. What is/ are the reaction intermediate(s)? k=Ae(RTEact) Arrhenius Equation lnk=(REact)(T1)+lnA ln(k2k1)=REact(T1T2T1T2) or ln(k2k1)=REact(T21T11) R=8.314KmolJ=0.0821molKLatm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


