Question: answer please :) Question 2 (1 point) How many grams of copper are required to produce 8.64 g of copper(ll) sulfate pentahydrate? The molar masses

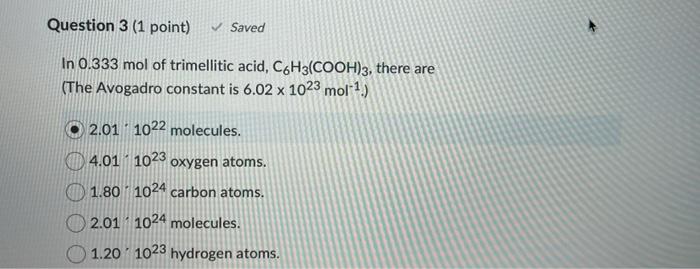
Question 2 (1 point) How many grams of copper are required to produce 8.64 g of copper(ll) sulfate pentahydrate? The molar masses of hydrogen, oxygen, sulfur and copper are 1.008, 16.00, 32.06, and 63.54 g.mol-1, respectively. Enter the numerical answer only with the correct number of significant figures. Your Answer: Your Answer Question 3 (1 point) Saved In 0.333 mol of trimellitic acid, C6H3(COOH)3, there are (The Avogadro constant is 6.02 x 1023 mol-1) 2.01 1022 molecules. 4.01 1023 oxygen atoms. 1.80 1024 carbon atoms. 2.01 1024 molecules. 1.20 1023 hydrogen atoms
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


