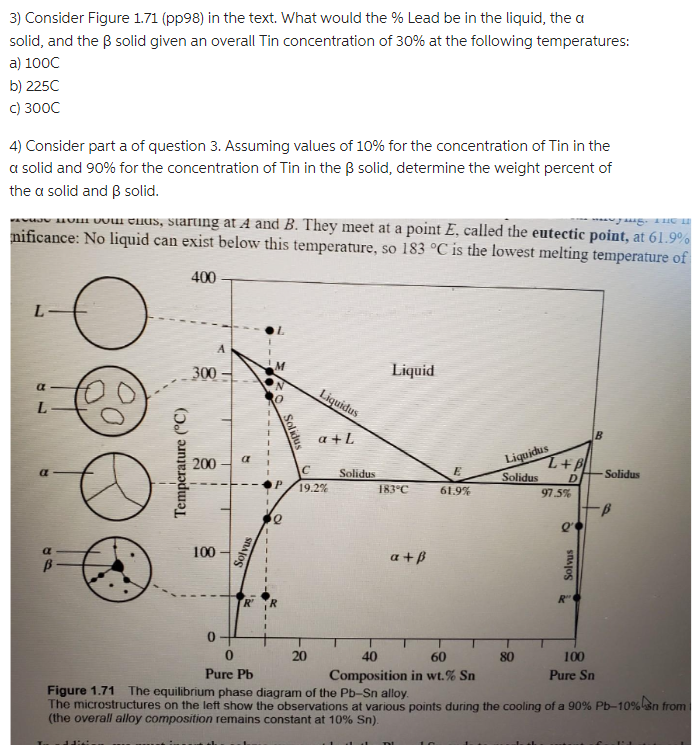
Question: ANSWER QUESTION 4 PLEASE AND THANK YOU 3) Consider Figure 1.71 (pp98) in the text. What would the % Lead be in the liquid, the
ANSWER QUESTION 4 PLEASE AND THANK YOU
3) Consider Figure 1.71 (pp98) in the text. What would the \% Lead be in the liquid, the a solid, and the solid given an overall Tin concentration of 30% at the following temperatures: a) 100C b) 225C c) 300C 4) Consider part a of question 3. Assuming values of 10% for the concentration of Tin in the a solid and 90% for the concentration of Tin in the solid, determine the weight percent of the solid and solid. nificance Nour enus, starung at A and B. They meet at point E, called the eutectic point, at 61.9 nificance: No liquid can exist below this temperature, so 183C is the lowest melting temperature of (the overall alloy composition remains constant at 10%Sn )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


